Blood transfusion and breast milk transmission of cytomegalovirus in very low-birth-weight infants: a prospective cohort study
- PMID: 25243446
- PMCID: PMC4392178
- DOI: 10.1001/jamapediatrics.2014.1360
Blood transfusion and breast milk transmission of cytomegalovirus in very low-birth-weight infants: a prospective cohort study
Abstract
Importance: Postnatal cytomegalovirus (CMV) infection can cause serious morbidity and mortality in very low-birth-weight (VLBW) infants. The primary sources of postnatal CMV infection in this population are breast milk and blood transfusion. The current risks attributable to these vectors, as well as the efficacy of approaches to prevent CMV transmission, are poorly characterized.
Objective: To estimate the risk of postnatal CMV transmission from 2 sources: (1) transfusion of CMV-seronegative and leukoreduced blood and (2) maternal breast milk.
Design, setting, and participants: A prospective, multicenter birth-cohort study was conducted from January 2010 to June 2013 at 3 neonatal intensive care units (2 academically affiliated and 1 private) in Atlanta, Georgia. Cytomegalovirus serologic testing of enrolled mothers was performed to determine their status. Cytomegalovirus nucleic acid testing (NAT) of transfused blood components and breast milk was performed to identify sources of CMV transmission. A total of 539 VLBW infants (birth weight, ≤ 1500 g) who had not received a blood transfusion were enrolled, with their mothers (n = 462), within 5 days of birth. The infants underwent serum and urine CMV NAT at birth to evaluate congenital infection and surveillance CMV NAT at 5 additional intervals between birth and 90 days, discharge, or death.
Exposures: Blood transfusion and breast milk feeding.
Main outcomes and measures: Cumulative incidence of postnatal CMV infection, detected by serum or urine NAT.
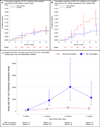
Results: The seroprevalence of CMV among the 462 enrolled mothers was 76.2% (n = 352). Among the 539 VLBW infants, the cumulative incidence of postnatal CMV infection at 12 weeks was 6.9% (95% CI, 4.2%-9.2%); 5 of 29 infants (17.2%) with postnatal CMV infection developed symptomatic disease or died. A total of 2061 transfusions were administered among 57.5% (n = 310) of the infants; none of the CMV infections was linked to transfusion, resulting in a CMV infection incidence of 0.0% (95% CI, 0.0%-0.3%) per unit of CMV-seronegative and leukoreduced blood. Twenty-seven of 28 postnatal infections occurred among infants fed CMV-positive breast milk (12-week incidence, 15.3%; 95% CI, 9.3%-20.2%).
Conclusions and relevance: Transfusion of CMV-seronegative and leukoreduced blood products effectively prevents transmission of CMV to VLBW infants. Among infants whose care is managed with this transfusion approach, maternal breast milk is the primary source of postnatal CMV infection.
Trial registration: clinicaltrials.gov Identifier: NCT00907686.
Conflict of interest statement
Figures

References
-
- Yeager AS, Grumet FC, Hafleigh EB, Arvin AM, Bradley JS, Prober CG. Prevention of transfusion-acquired cytomegalovirus infections in newborn infants. J Pediatr. 1981;98(2):281–287. - PubMed
-
- Gilbert GL, Hayes IL, Hudson IL, James J. Prevention of transfusion-acquired cytomegalovirus infection in infants by blood filtration to remove leukocytes.: Neonatal Cytomegalovirus Infection study group. Lancet. 1989;1(8649):1228–1231. - PubMed
-
- Eisenfeld L, Silver H, McLaughlin J, Klevjer-Anderson Prevention of transfusion-associated cytomegalovirus infection in neonatal patients by the removal of white cells from blood. Transfusion. 1992;32(3):205–209. - PubMed
-
- Josephson CD, Roback JR. New insights for preventing transfusion-transmitted cytomegalovirus and other white blood cell-associated viral infections. Transfusion. 2013;53(10):2112–2116. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

