Chemotherapy enhances cross-presentation of nuclear tumor antigens
- PMID: 25243472
- PMCID: PMC4171494
- DOI: 10.1371/journal.pone.0107894
Chemotherapy enhances cross-presentation of nuclear tumor antigens
Abstract
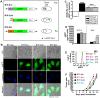
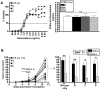
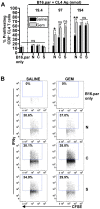
Cross-presentation of tumor antigen is essential for efficient priming of naïve CD8⁺ T lymphocytes and induction of effective anti-tumor immunity. We hypothesized that the subcellular location of a tumor antigen could affect the efficiency of cross-presentation, and hence the outcome of anti-tumor responses to that antigen. We compared cross-presentation of a nominal antigen expressed in the nuclear, secretory, or cytoplasmic compartments of B16 melanoma tumors. All tumors expressed similar levels of the antigen. The antigen was cross-presented from all compartments but when the concentration was low, nuclear antigen was less efficiently cross-presented than antigen from other cellular locations. The efficiency of cross-presentation of the nuclear antigen was improved following chemotherapy-induced tumor cell apoptosis and this correlated with an increase in the proportion of effector CTL. These data demonstrate that chemotherapy improves nuclear tumor antigen cross-presentation and could be important for anti-cancer immunotherapies that target nuclear antigens.
Conflict of interest statement
Figures

References
-
- Steer HJ, Lake RA, Nowak AK, Robinson BW (2010) Harnessing the immune response to treat cancer. Oncogene. - PubMed
-
- Segura E, Villadangos JA (2011) A modular and combinatorial view of the antigen cross-presentation pathway in dendritic cells. Traffic 12: 1677–1685. - PubMed
-
- Kurts C, Robinson BW, Knolle PA (2010) Cross-priming in health and disease. Nat Rev Immunol 10: 403–414. - PubMed
-
- Lake RA, Robinson BW (2005) Immunotherapy and chemotherapy–a practical partnership. Nat Rev Cancer 5: 397–405. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

