Diguanylate cyclase NicD-based signalling mechanism of nutrient-induced dispersion by Pseudomonas aeruginosa
- PMID: 25243483
- PMCID: PMC4227967
- DOI: 10.1111/mmi.12802
Diguanylate cyclase NicD-based signalling mechanism of nutrient-induced dispersion by Pseudomonas aeruginosa
Abstract
Dispersion enables the transition from the biofilm to the planktonic growth state in response to various cues. While several Pseudomonas aeruginosa proteins, including BdlA and the c-di-GMP phosphodiesterases DipA, RbdA, and NbdA, have been shown to be required for dispersion to occur, little is known about dispersion cue sensing and the signalling translating these cues into the modulation c-di-GMP levels to enable dispersion. Using glutamate-induced dispersion as a model, we report that dispersion-inducing nutrient cues are sensed via an outside-in signalling mechanism by the diguanylate cyclase NicD belonging to a family of seven transmembrane (7TM) receptors. NicD directly interacts with BdlA and the phosphodiesterase DipA, with NicD, BdlA, and DipA being part of the same pathway required for dispersion. Glutamate sensing by NicD results in NicD dephosphorylation and increased cyclase activity. Active NicD contributes to the non-processive proteolysis and activation of BdlA via phosphorylation and temporarily elevated c-di-GMP levels. BdlA, in turn, activates DipA, resulting in the overall reduction of c-di-GMP levels. Our results provide a basis for understanding the signalling mechanism based on NicD to induce biofilm dispersion that may be applicable to various biofilm-forming species and may have implications for the control of biofilm-related infections.
© 2014 John Wiley & Sons Ltd.
Figures



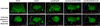
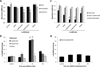


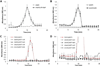

Comment in
-
Biofilm dispersal: deciding when it is better to travel.Mol Microbiol. 2014 Nov;94(4):747-50. doi: 10.1111/mmi.12797. Epub 2014 Sep 29. Mol Microbiol. 2014. PMID: 25223879
References
-
- Aldridge P, Paul R, Goymer P, Rainey P, Jenal U. Role of the GGDEF regulator PleD in polar development of Caulobacter crescentus . Mol. Microbiol. 2003;47:1695–1708. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

