Copper directs ATP7B to the apical domain of hepatic cells via basolateral endosomes
- PMID: 25243755
- PMCID: PMC4241365
- DOI: 10.1111/tra.12229
Copper directs ATP7B to the apical domain of hepatic cells via basolateral endosomes
Erratum in
- Traffic. 2015 Jan;16(1):99
Abstract
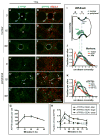
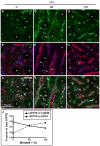
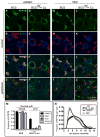
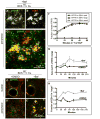
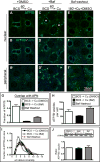
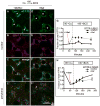
Physiologic Cu levels regulate the intracellular location of the Cu ATPase ATP7B. Here, we determined the routes of Cu-directed trafficking of endogenous ATP7B in the polarized hepatic cell line WIF-B and in the liver in vivo. Copper (10 µm) caused ATP7B to exit the trans-Golgi network (TGN) in vesicles, which trafficked via large basolateral endosomes to the apical domain within 1 h. Although perturbants of luminal acidification had little effect on the TGN localization of ATP7B in low Cu, they blocked delivery to the apical membrane in elevated Cu. If the vesicular proton-pump inhibitor bafilomycin-A1 (Baf) was present with Cu, ATP7B still exited the TGN, but accumulated in large endosomes located near the coverslip, in the basolateral region. Baf washout restored ATP7B trafficking to the apical domain. If ATP7B was staged apically in high Cu, Baf addition promoted the accumulation of ATP7B in subapical endosomes, indicating a blockade of apical recycling, with concomitant loss of ATP7B at the apical membrane. The retrograde pathway to the TGN, induced by Cu removal, was far less affected by Baf than the anterograde (Cu-stimulated) case. Overall, loss of acidification-impaired Cu-regulated trafficking of ATP7B at two main sites: (i) sorting and exit from large basolateral endosomes and (ii) recycling via endosomes near the apical membrane.
Keywords: Wilson's disease; bafliomycin-A1; bile canaliculus; endosomes; hepatocytes; polarity; proton ATPase; retromer.
© 2014 The Authors. Traffic published by John Wiley & Sons Ltd.
Figures




References
-
- Scheiber I, Dringen R, Mercer JF. Copper: effects of deficiency and overload. Metal Ions in Life Sciences. 2013;13:359–387. - PubMed
-
- Lutsenko S, Barnes NL, Bartee MY, Dmitriev OY. Function and regulation of human copper-transporting ATPases. Physiological Reviews. 2007;87(3):1011–1046. - PubMed
-
- Schaefer M, Hopkins RG, Failla ML, Gitlin JD. Hepatocyte-specific localization and copperdependent trafficking of the Wilson's disease protein in the liver. The American Journal of Physiology. 1999;276(3 Pt 1):G639–646. - PubMed
-
- Braiterman LT, Murthy A, Jayakanthan S, Nyasae L, Tzeng E, Gromadzka G, Woolf TB, Lutsenko S, Hubbard AL. Distinct phenotype of a Wilson disease mutation reveals a novel trafficking determinant in the copper transporter ATP7B. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(14):E1364–1373. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

