Lactulose vs polyethylene glycol 3350--electrolyte solution for treatment of overt hepatic encephalopathy: the HELP randomized clinical trial
- PMID: 25243839
- PMCID: PMC5609454
- DOI: 10.1001/jamainternmed.2014.4746
Lactulose vs polyethylene glycol 3350--electrolyte solution for treatment of overt hepatic encephalopathy: the HELP randomized clinical trial
Abstract
Importance: Hepatic encephalopathy (HE) is a common cause of hospitalization in patients with cirrhosis. Pharmacologic treatment for acute (overt) HE has remained the same for decades.
Objective: To compare polyethylene glycol 3350-electrolyte solution (PEG) and lactulose treatments in patients with cirrhosis admitted to the hospital for HE. We hypothesized that rapid catharsis of the gut using PEG may resolve HE more effectively than lactulose.
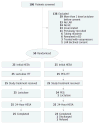
Design, setting, and participants: The HELP (Hepatic Encephalopathy: Lactulose vs Polyethylene Glycol 3350-Electrolyte Solution) study is a randomized clinical trial in an academic tertiary hospital of 50 patients with cirrhosis (of 186 screened) admitted for HE.
Interventions: Participants were block randomized to receive treatment with PEG, 4-L dose (n = 25), or standard-of-care lactulose (n = 25) during hospitalization.
Main outcomes and measures: The primary end point was an improvement of 1 or more in HE grade at 24 hours, determined using the hepatic encephalopathy scoring algorithm (HESA), ranging from 0 (normal clinical and neuropsychological assessments) to 4 (coma). Secondary outcomes included time to HE resolution and overall length of stay.
Results: A total of 25 patients were randomized to each treatment arm. Baseline clinical features at admission were similar in the groups. Thirteen of 25 patients in the standard therapy arm (52%) had an improvement of 1 or more in HESA score, thus meeting the primary outcome measure, compared with 21 of 23 evaluated patients receiving PEG (91%) (P < .01); 1 patient was discharged before final analysis and 1 refused participation. The mean (SD) HESA score at 24 hours for patients receiving standard therapy changed from 2.3 (0.9) to 1.6 (0.9) compared with a change from 2.3 (0.9) to 0.9 (1.0) for the PEG-treated groups (P = .002). The median time for HE resolution was 2 days for standard therapy and 1 day for PEG (P = .01). Adverse events were uncommon, and none was definitely study related.
Conclusions and relevance: PEG led to more rapid HE resolution than standard therapy, suggesting that PEG may be superior to standard lactulose therapy in patients with cirrhosis hospitalized for acute HE.
Trial registration: clinicaltrials.gov Identifier: NCT01283152.
Conflict of interest statement
Figures
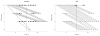

Comment in
-
Polyethylene glycol for hepatic encephalopathy: a new solution to purge an old problem?JAMA Intern Med. 2014 Nov;174(11):1734-5. doi: 10.1001/jamainternmed.2014.3501. JAMA Intern Med. 2014. PMID: 25244573 No abstract available.
-
A gut solution for hepatic encephalopathy.Hepatology. 2015 Jun;61(6):2107-009. doi: 10.1002/hep.27784. Epub 2015 Apr 22. Hepatology. 2015. PMID: 25777043 No abstract available.
-
Lactulose vs Polyethylene Glycol for Treatment of Hepatic Encephalopathy.JAMA Intern Med. 2015 May;175(5):867-8. doi: 10.1001/jamainternmed.2015.0321. JAMA Intern Med. 2015. PMID: 25938320 No abstract available.
-
Lactulose vs Polyethylene Glycol for Treatment of Hepatic Encephalopathy.JAMA Intern Med. 2015 May;175(5):868. doi: 10.1001/jamainternmed.2015.0331. JAMA Intern Med. 2015. PMID: 25938321 No abstract available.
-
Lactulose vs Polyethylene Glycol for Treatment of Hepatic Encephalopathy-Reply.JAMA Intern Med. 2015 May;175(5):868-9. doi: 10.1001/jamainternmed.2015.0334. JAMA Intern Med. 2015. PMID: 25938322 No abstract available.
-
[Overt hepatic encephalopathy: extension of therapeutic armamentarium by old friends?].Z Gastroenterol. 2015 Jul;53(7):724-5. doi: 10.1055/s-0034-1399696. Epub 2015 Jul 13. Z Gastroenterol. 2015. PMID: 26167699 German. No abstract available.
References
-
- Ferenci P, Lockwood A, Mullen K, Tarter R, Weissenborn K, Blei AT. Hepatic encephalopathy—definition, nomenclature, diagnosis, and quantification: final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology. 2002;35(3):716–721. - PubMed
-
- Blei AT, Córdoba J Practice Parameters Committee of the American College of Gastroenterology. Hepatic encephalopathy. Am J Gastroenterol. 2001;96(7):1968–1976. - PubMed
-
- Als-Nielsen B, Gluud LL, Gluud C. Nonabsorbable disaccharides for hepatic encephalopathy. Cochrane Database Syst Rev. 2004;(2):CD003044. - PubMed
-
- Elkington SG, Floch MH, Conn HO. Lactulose in the treatment of chronic portal-systemic encephalopathy: a double-blind clinical trial. N Engl J Med. 1969;281(8):408–412. - PubMed
-
- Bircher J, Müller J, Guggenheim P, Haemmerli UP. Treatment of chronic portal-systemic encephalopathy with lactulose. Lancet. 1966;1(7443):890–892. - PubMed

