Deep sequencing reveals stepwise mutation acquisition in paroxysmal nocturnal hemoglobinuria
- PMID: 25244093
- PMCID: PMC4191017
- DOI: 10.1172/JCI74747
Deep sequencing reveals stepwise mutation acquisition in paroxysmal nocturnal hemoglobinuria
Abstract
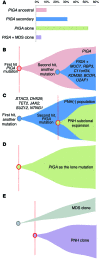
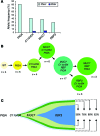
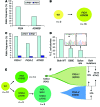
Paroxysmal nocturnal hemoglobinuria (PNH) is a nonmalignant clonal disease of hematopoietic stem cells that is associated with hemolysis, marrow failure, and thrombophilia. PNH has been considered a monogenic disease that results from somatic mutations in the gene encoding PIGA, which is required for biosynthesis of glycosylphosphatidylinisotol-anchored (GPI-anchored) proteins. The loss of certain GPI-anchored proteins is hypothesized to provide the mutant clone with an extrinsic growth advantage, but some features of PNH argue that there are intrinsic drivers of clonal expansion. Here, we performed whole-exome sequencing of paired PNH+ and PNH- fractions on samples taken from 12 patients as well as targeted deep sequencing of an additional 36 PNH patients. We identified additional somatic mutations that resulted in a complex hierarchical clonal architecture, similar to that observed in myeloid neoplasms. In addition to mutations in PIGA, mutations were found in genes known to be involved in myeloid neoplasm pathogenesis, including TET2, SUZ12, U2AF1, and JAK2. Clonal analysis indicated that these additional mutations arose either as a subclone within the PIGA-mutant population, or prior to PIGA mutation. Together, our data indicate that in addition to PIGA mutations, accessory genetic events are frequent in PNH, suggesting a stepwise clonal evolution derived from a singular stem cell clone.
Figures

Comment in
-
The mutational landscape of paroxysmal nocturnal hemoglobinuria revealed: new insights into clonal dominance.J Clin Invest. 2014 Oct;124(10):4227-30. doi: 10.1172/JCI77984. Epub 2014 Sep 17. J Clin Invest. 2014. PMID: 25244089 Free PMC article.
References
-
- Brodsky RA. Paroxysmal nocturnal hemoglobinuria: stem cells and clonality. Hematology Am Soc Hematol Educ Program. 2008:111–115. - PubMed
-
- Maciejewski JP, Sloand EM, Sato T, Anderson S, Young NS. Impaired hematopoiesis in paroxysmal nocturnal hemoglobinuria/aplastic anemia is not associated with a selective proliferative defect in the glycosylphosphatidylinositol-anchored protein-deficient clone. Blood. 1997;89(4):1173–1181. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

