B7-H1-expressing antigen-presenting cells mediate polarization of protumorigenic Th22 subsets
- PMID: 25244097
- PMCID: PMC4191045
- DOI: 10.1172/JCI74381
B7-H1-expressing antigen-presenting cells mediate polarization of protumorigenic Th22 subsets
Abstract
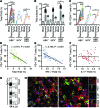
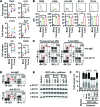
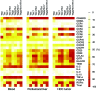
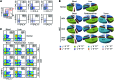
Classical IL-22-producing T helper cells (Th22 cells) mediate inflammatory responses independently of IFN-γ and IL-17; however, nonclassical Th22 cells have been recently identified and coexpress IFN-γ and/or IL-17 along with IL-22. Little is known about how classical and nonclassical Th22 subsets in human diseases are regulated. Here, we used samples of human blood, normal and peritumoral liver, and hepatocellular carcinoma (HCC) to delineate the phenotype, distribution, generation, and functional relevance of various Th22 subsets. Three nonclassical Th22 subsets constituted the majority of all Th22 cells in human liver and HCC tissues, although the classical Th22 subset was predominant in blood. Monocytes activated by TLR2 and TLR4 agonists served as the antigen-presenting cells (APCs) that most efficiently triggered the expansion of nonclassical Th22 subsets from memory T cells and classical Th22 subsets from naive T cells. Moreover, B7-H1-expressing monocytes skewed Th22 polarization away from IFN-γ and toward IL-17 through interaction with programmed death 1 (PD-1), an effect that can create favorable conditions for in vivo aggressive cancer growth and angiogenesis. Our results provide insight into the selective modulation of Th22 subsets and suggest that strategies to influence functional activities of inflammatory cells may benefit anticancer therapy.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

