Leukemic stem cell frequency: a strong biomarker for clinical outcome in acute myeloid leukemia
- PMID: 25244440
- PMCID: PMC4171508
- DOI: 10.1371/journal.pone.0107587
Leukemic stem cell frequency: a strong biomarker for clinical outcome in acute myeloid leukemia
Abstract
Introduction: Treatment failure in acute myeloid leukemia is probably caused by the presence of leukemia initiating cells, also referred to as leukemic stem cells, at diagnosis and their persistence after therapy. Specific identification of leukemia stem cells and their discrimination from normal hematopoietic stem cells would greatly contribute to risk stratification and could predict possible relapses.
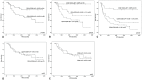
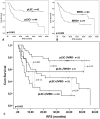
Results: For identification of leukemic stem cells, we developed flow cytometric methods using leukemic stem cell associated markers and newly-defined (light scatter) aberrancies. The nature of the putative leukemic stem cells and normal hematopoietic stem cells, present in the same patient's bone marrow, was demonstrated in eight patients by the presence or absence of molecular aberrancies and/or leukemic engraftment in NOD-SCID IL-2Rγ-/- mice. At diagnosis (n=88), the frequency of the thus defined neoplastic part of CD34+CD38- putative stem cell compartment had a strong prognostic impact, while the neoplastic parts of the CD34+CD38+ and CD34- putative stem cell compartments had no prognostic impact at all. After different courses of therapy, higher percentages of neoplastic CD34+CD38- cells in complete remission strongly correlated with shorter patient survival (n=91). Moreover, combining neoplastic CD34+CD38- frequencies with frequencies of minimal residual disease cells (n=91), which reflect the total neoplastic burden, revealed four patient groups with different survival.
Conclusion and perspective: Discrimination between putative leukemia stem cells and normal hematopoietic stem cells in this large-scale study allowed to demonstrate the clinical importance of putative CD34+CD38- leukemia stem cells in AML. Moreover, it offers new opportunities for the development of therapies directed against leukemia stem cells, that would spare normal hematopoietic stem cells, and, moreover, enables in vivo and ex vivo screening for potential efficacy and toxicity of new therapies.
Conflict of interest statement
Figures





References
-
- Valent P, Bonnet D, De MR, Lapidot T, Copland M, et al. (2012) Cancer stem cell definitions and terminology: the devil is in the details. Nat Rev Cancer 12: 767–775. - PubMed
-
- Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, et al. (1994) A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature 367: 645–648. - PubMed
-
- Bonnet D, Dick JE (1997) Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nature medicine 3: 730–737. - PubMed
-
- Dick JE (2008) Stem cell concepts renew cancer research. Blood 112: 4793–4807. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

