Germ cell-specific targeting of DICER or DGCR8 reveals a novel role for endo-siRNAs in the progression of mammalian spermatogenesis and male fertility
- PMID: 25244517
- PMCID: PMC4171096
- DOI: 10.1371/journal.pone.0107023
Germ cell-specific targeting of DICER or DGCR8 reveals a novel role for endo-siRNAs in the progression of mammalian spermatogenesis and male fertility
Abstract
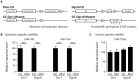
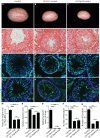
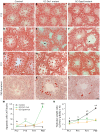
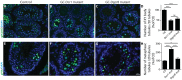
Small non-coding RNAs act as critical regulators of gene expression and are essential for male germ cell development and spermatogenesis. Previously, we showed that germ cell-specific inactivation of Dicer1, an endonuclease essential for the biogenesis of micro-RNAs (miRNAs) and endogenous small interfering RNAs (endo-siRNAs), led to complete male infertility due to alterations in meiotic progression, increased spermatocyte apoptosis and defects in the maturation of spermatozoa. To dissect the distinct physiological roles of miRNAs and endo-siRNAs in spermatogenesis, we compared the testicular phenotype of mice with Dicer1 or Dgcr8 depletion in male germ cells. Dgcr8 mutant mice, which have a defective miRNA pathway while retaining an intact endo-siRNA pathway, were also infertile and displayed similar defects, although less severe, to Dicer1 mutant mice. These included cumulative defects in meiotic and haploid phases of spermatogenesis, resulting in oligo-, terato-, and azoospermia. In addition, we found by RNA sequencing of purified spermatocytes that inactivation of Dicer1 and the resulting absence of miRNAs affected the fine tuning of protein-coding gene expression by increasing low level gene expression. Overall, these results emphasize the essential role of miRNAs in the progression of spermatogenesis, but also indicate a role for endo-siRNAs in this process.
Conflict of interest statement
Figures
Similar articles
-
Dicer1 depletion in male germ cells leads to infertility due to cumulative meiotic and spermiogenic defects.PLoS One. 2011;6(10):e25241. doi: 10.1371/journal.pone.0025241. Epub 2011 Oct 5. PLoS One. 2011. PMID: 21998645 Free PMC article.
-
The role of Dicer1 in the male reproductive tract.Asian J Androl. 2015 Sep-Oct;17(5):737-41. doi: 10.4103/1008-682X.155542. Asian J Androl. 2015. PMID: 25994652 Free PMC article. Review.
-
Dicer is required for haploid male germ cell differentiation in mice.PLoS One. 2011;6(9):e24821. doi: 10.1371/journal.pone.0024821. Epub 2011 Sep 16. PLoS One. 2011. PMID: 21949761 Free PMC article.
-
The RNase III enzyme DROSHA is essential for microRNA production and spermatogenesis.J Biol Chem. 2012 Jul 20;287(30):25173-90. doi: 10.1074/jbc.M112.362053. Epub 2012 Jun 4. J Biol Chem. 2012. PMID: 22665486 Free PMC article.
-
Small RNAs in spermatogenesis.Mol Cell Endocrinol. 2014 Jan 25;382(1):498-508. doi: 10.1016/j.mce.2013.04.015. Epub 2013 Apr 28. Mol Cell Endocrinol. 2014. PMID: 23632103 Review.
Cited by
-
From Sperm Motility to Sperm-Borne microRNA Signatures: New Approaches to Predict Male Fertility Potential.Front Cell Dev Biol. 2020 Aug 21;8:791. doi: 10.3389/fcell.2020.00791. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32974342 Free PMC article. Review.
-
The roles of microRNAs and siRNAs in mammalian spermatogenesis.Development. 2016 Sep 1;143(17):3061-73. doi: 10.1242/dev.136721. Development. 2016. PMID: 27578177 Free PMC article. Review.
-
Integrated Analysis of Long Non-Coding RNA and mRNA Expression Profiles in Testes of Calves and Sexually Mature Wandong Bulls (Bos taurus).Animals (Basel). 2021 Jul 5;11(7):2006. doi: 10.3390/ani11072006. Animals (Basel). 2021. PMID: 34359134 Free PMC article.
-
Transposable Element Bm1645 is a Source of BmAGO2-associated Small RNAs that affect its expression in Bombyx mori.BMC Genomics. 2017 Feb 23;18(1):201. doi: 10.1186/s12864-017-3598-5. BMC Genomics. 2017. PMID: 28231766 Free PMC article.
-
Of rodents and ruminants: a comparison of small noncoding RNA requirements in mouse and bovine reproduction.J Anim Sci. 2021 Mar 1;99(3):skaa388. doi: 10.1093/jas/skaa388. J Anim Sci. 2021. PMID: 33677580 Free PMC article.
References
-
- Eddy EM (2002) Male germ cell gene expression. Recent Prog Horm Res 57: 103–128. - PubMed
-
- He Z, Kokkinaki M, Pant D, Gallicano GI, Dym M (2009) Small RNA molecules in the regulation of spermatogenesis. Reproduction 137: 901–911. - PubMed
-
- Kim VN, Han J, Siomi MC (2009) Biogenesis of small RNAs in animals. Nat Rev Mol Cell Biol 10: 126–139. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

