Hijacking bacterial glycosylation for the production of glycoconjugates, from vaccines to humanised glycoproteins
- PMID: 25244672
- PMCID: PMC4724881
- DOI: 10.1111/jphp.12321
Hijacking bacterial glycosylation for the production of glycoconjugates, from vaccines to humanised glycoproteins
Abstract
Objectives: Glycosylation or the modification of a cellular component with a carbohydrate moiety has been demonstrated in all three domains of life as a basic post-translational process important in a range of biological processes. This review will focus on the latest studies attempting to exploit bacterial N-linked protein glycosylation for glycobiotechnological applications including glycoconjugate vaccine and humanised glycoprotein production. The challenges that remain for these approaches to reach full biotechnological maturity will be discussed.
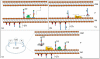
Key findings: Oligosaccharyltransferase-dependent N-linked glycosylation can be exploited to make glycoconjugate vaccines against bacterial pathogens. Few technical limitations remain, but it is likely that the technologies developed will soon be considered a cost-effective and flexible alternative to current chemical-based methods of vaccine production. Some highlights from current glycoconjugate vaccines developed using this in-vivo production system include a vaccine against Shigella dysenteriae O1 that has passed phase 1 clinical trials, a vaccine against the tier 1 pathogen Francisella tularensis that has shown efficacy in mice and a vaccine against Staphylococcus aureus serotypes 5 and 8. Generation of humanised glycoproteins within bacteria was considered impossible due to the distinct nature of glycan modification in eukaryotes and prokaryotes. We describe the method used to overcome this conundrum to allow engineering of a eukaryotic pentasaccharide core sugar modification within Escherichia coli. This core was assembled by combining the function of the initiating transferase WecA, several Alg genes from Saccharomyces cerevisiae and the oligosaccharyltransferase function of the Campylobacter jejuni PglB. Further exploitation of a cytoplasmic N-linked glycosylation system found in Actinobacillus pleuropneumoniae where the central enzyme is known as N-linking glycosyltransferase has overcome some of the limitations demonstrated by the oligosaccharyltransferase-dependent system.
Summary: Characterisation of the first bacterial N-linked glycosylation system in the human enteropathogen Campylobacter jejuni has led to substantial biotechnological applications. Alternative methods for glycoconjugate vaccine production have been developed using this N-linked system. Vaccines against both Gram-negative and Gram-positive organisms have been developed, and efficacy testing has thus far demonstrated that the vaccines are safe and that robust immune responses are being detected. These are likely to complement and reduce the cost of current technologies thus opening new avenues for glycoconjugate vaccines. These new markets could potentially include glycoconjugate vaccines tailored specifically for animal vaccination, which has until today thought to be non-viable due to the cost of current in-vitro chemical conjugation methods. Utilisation of N-linked glycosylation to generate humanised glycoproteins is also close to becoming reality. This 'bottom up' assembly mechanism removes the heterogeneity seen in current humanised products. The majority of developments reported in this review exploit a single N-linked glycosylation system from Campylobacter jejuni; however, alternative N-linked glycosylation systems have been discovered which should help to overcome current technical limitations and perhaps more systems remain to be discovered. The likelihood is that further glycosylation systems exist and are waiting to be exploited.
Keywords: PglB; glycoconjugates; humanised glycoproteins; vaccines.
© 2014 Royal Pharmaceutical Society.
Figures


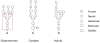

References
-
- Coutinho A, Moller G. B cell mitogenic properties of thymus-independent antigens. Nat New Biol. 1973;245:12–14. - PubMed
-
- Timens W, et al. Immaturity of the human splenic marginal zone in infancy. Possible contribution to the deficient infant immune response. J Immunol. 1989;143:3200–3206. - PubMed
-
- Shi Y, et al. Regulation of aged humoral immune defense against pneumococcal bacteria by IgM memory B cell. J Immunol. 2005;175:3262–3267. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

