HMGA1 and HMGA2 expression and comparative analyses of HMGA2, Lin28 and let-7 miRNAs in oral squamous cell carcinoma
- PMID: 25245141
- PMCID: PMC4190370
- DOI: 10.1186/1471-2407-14-694
HMGA1 and HMGA2 expression and comparative analyses of HMGA2, Lin28 and let-7 miRNAs in oral squamous cell carcinoma
Abstract
Background: Humans and dogs are affected by squamous cell carcinomas of the oral cavity (OSCC) in a considerably high frequency. The high mobility group A2 (HMGA2) protein was found to be highly expressed in human OSCC and its expression was suggested to act as a useful predictive and prognostic tool in clinical management of oral carcinomas. Herein the expression of HMGA2 and its sister gene HMGA1 were analysed within human and canine OSCC samples. Additionally, the HMGA negatively regulating miRNAs of the let-7 family as well as the let-7 regulating gene Lin28 were also comparatively analysed. Deregulations of either one of these members could affect the progression of human and canine OSCC.
Methods: Expression levels of HMGA1, HMGA2, Lin28, let-7a and mir-98 were analysed via relative qPCR in primary human and canine OSCC, thereof derived cell lines and non-neoplastic samples. Additionally, comparative HMGA2 protein expression was analysed by immunohistochemistry.
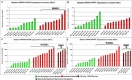
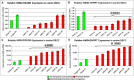
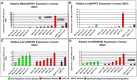
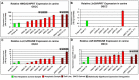
Results: In both species, a significant up-regulation of the HMGA2 gene was found within the neoplastic samples while HMGA1 expression did not show significant deregulations. Comparative analyses showed down-regulation of mir-98 in human samples and up-regulation of let-7a and mir-98 in canine neoplastic samples. HMGA2 immunostainings showed higher intensities within the invasive front of the tumours than in the centre of the tumour in both species.
Conclusions: HMGA2 could potentially serve as tumour marker in both species while HMGA1 might play a minor role in OSCC progression. Comparative studies indicate an inverse correlation of HMGA2 and mir-98 expression in human samples whereas in dogs no such characteristic could be found.
Figures


Similar articles
-
HMGA2, but not HMGA1, is overexpressed in human larynx carcinomas.Histopathology. 2018 Jun;72(7):1102-1114. doi: 10.1111/his.13456. Epub 2018 Mar 9. Histopathology. 2018. PMID: 29266325
-
Dysregulation of TCONS_00006091 contributes to the elevated risk of oral squamous cell carcinoma by upregulating SNAI1, IRS and HMGA2.Sci Rep. 2024 Apr 26;14(1):9616. doi: 10.1038/s41598-024-60310-4. Sci Rep. 2024. PMID: 38671227 Free PMC article.
-
HMGA2 is down-regulated by microRNA let-7 and associated with epithelial-mesenchymal transition in oesophageal squamous cell carcinomas of Kazakhs.Histopathology. 2014 Sep;65(3):408-17. doi: 10.1111/his.12401. Epub 2014 May 12. Histopathology. 2014. PMID: 24612219
-
HMGA2: A pituitary tumour subtype-specific oncogene?Mol Cell Endocrinol. 2010 Sep 15;326(1-2):19-24. doi: 10.1016/j.mce.2010.03.019. Epub 2010 Mar 27. Mol Cell Endocrinol. 2010. PMID: 20347930 Review.
-
Evaluating the role of microRNAs alterations in oral squamous cell carcinoma.Gene. 2020 Oct 5;757:144936. doi: 10.1016/j.gene.2020.144936. Epub 2020 Jul 5. Gene. 2020. PMID: 32640301 Review.
Cited by
-
Integrated network analysis and logistic regression modeling identify stage-specific genes in Oral Squamous Cell Carcinoma.BMC Med Genomics. 2015 Jul 16;8:39. doi: 10.1186/s12920-015-0114-0. BMC Med Genomics. 2015. PMID: 26179909 Free PMC article.
-
Let-7 inhibits self-renewal of hepatocellular cancer stem-like cells through regulating the epithelial-mesenchymal transition and the Wnt signaling pathway.BMC Cancer. 2016 Nov 8;16(1):863. doi: 10.1186/s12885-016-2904-y. BMC Cancer. 2016. PMID: 27821157 Free PMC article.
-
The Prominent Role of HMGA Proteins in the Early Management of Gastrointestinal Cancers.Biomed Res Int. 2019 Oct 13;2019:2059516. doi: 10.1155/2019/2059516. eCollection 2019. Biomed Res Int. 2019. PMID: 31737655 Free PMC article. Review.
-
Circulating small non-coding RNA signature in head and neck squamous cell carcinoma.Oncotarget. 2015 Aug 7;6(22):19246-63. doi: 10.18632/oncotarget.4266. Oncotarget. 2015. PMID: 26057471 Free PMC article.
-
Genome-wide haplotype association analysis identifies SERPINB9, SERPINE2, GAK, and HSP90B1 as novel risk genes for oral squamous cell carcinoma.Tumour Biol. 2016 Feb;37(2):1845-51. doi: 10.1007/s13277-015-3965-2. Epub 2015 Aug 30. Tumour Biol. 2016. PMID: 26318431
References
-
- Withrow S, Vail D. Withrow and MacEwen’s Small Animal Clinical Oncology. 4. St. Louis: Saunders Elsevier; 2007.
-
- Mallet Y, Avalos N, Le Ridant AM, Gangloff P, Moriniere S, Rame JP, Poissonnet G, Makeieff M, Cosmidis A, Babin E, Barry B, Fournier C. Head and neck cancer in young people: a series of 52 SCCs of the oral tongue in patients aged 35 years or less. Acta Otolaryngol. 2009;129(12):1503–1508. doi: 10.3109/00016480902798343. - DOI - PubMed
-
- Kokemueller H, Rana M, Rublack J, Eckardt A, Tavassol F, Schumann P, Lindhorst D, Ruecker M, Gellrich NC. The Hannover experience: surgical treatment of tongue cancer–a clinical retrospective evaluation over a 30 years period. Head Neck Oncol. 2011;3:27. doi: 10.1186/1758-3284-3-27. - DOI - PMC - PubMed
Pre-publication history
-
- The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1471-2407/14/694/prepub
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

