Increased exposure of norethindrone in HIV+ women treated with ritonavir-boosted atazanavir therapy
- PMID: 25245190
- PMCID: PMC4268426
- DOI: 10.1016/j.contraception.2014.08.009
Increased exposure of norethindrone in HIV+ women treated with ritonavir-boosted atazanavir therapy
Abstract
Objective: Pharmacokinetics of norethindrone in combination oral contraceptive regimen are well described among HIV+ women treated with ritonavir-boosted protease inhibitor therapies; however, such characterization is lacking in women using progestin-only contraception. Our objective is to characterize pharmacokinetics of norethindrone in HIV+ women using ritonavir-boosted atazanavir treatment during progestin-only contraceptive regimens.
Study design: An open-label, prospective, nonrandomized trial to characterize the pharmacokinetics of norethindrone in HIV+ women receiving ritonavir-boosted atazanavir (n=10; treatment group) and other antiretroviral therapy known to not alter norethindrone levels (n=17; control group) was conducted. Following informed consent, women were instructed to take a single daily fixed oral dose of 0.35 mg norethindrone and 300 mg/100 mg atazanavir/ritonavir for 22 days. On day 22, serial blood samples were collected by venous catheter at 0, 1, 2, 3, 4, 6, 8, 12, 24, 48 and 72 h. Whole blood was processed to collect serum and stored at -20°C until later analysis using radioimmunoassay. Pharmacokinetic parameters were estimated using noncompartmental method.
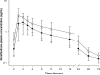
Results: In the treatment group, compared to the control group, an increase in area under the curve₀₋₂₄ (16.69 h*ng/mL vs. 25.20 h*ng/mL; p<.05) and maximum serum concentration (2.09 ng/mL vs. 3.19 ng/mL; p<.05), decrease (25%-40%) in apparent volume of distribution and apparent clearance, and unaltered half-life were observed.
Conclusion(s): Our findings suggest that progestin-only contraceptives, unlike combination oral contraceptives, benefit from drug-drug interaction and achieve higher levels of exposure. Further studies are needed to establish whether pharmacokinetic interaction leads to favorable clinical outcomes.
Implications: Norethindrone-based progestin-only contraceptives, unlike combination oral contraceptives, exhibit greater drug exposure when co-administered with ritonavir-boosted atazanavir regimen and thus may not warrant a category 3 designation by the World Health Organization. Prospective studies are needed to confirm whether pharmacokinetic interaction results in favorable clinical outcomes.
Trial registration: ClinicalTrials.gov NCT01667978.
Keywords: Norethindrone; Pharmacokinetics; Progestin-only contraception; Ritonavir.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures
Similar articles
-
Effect of protease inhibitors on steady-state pharmacokinetics of oral norethindrone contraception in HIV-infected women.J Acquir Immune Defic Syndr. 2014 Jan 1;65(1):72-7. doi: 10.1097/QAI.0b013e3182a9b3f1. J Acquir Immune Defic Syndr. 2014. PMID: 24025339 Free PMC article. Clinical Trial.
-
Tenofovir comedication does not impair the steady-state pharmacokinetics of ritonavir-boosted atazanavir in HIV-1-infected adults.Eur J Clin Pharmacol. 2007 Oct;63(10):935-40. doi: 10.1007/s00228-007-0344-y. Epub 2007 Jul 31. Eur J Clin Pharmacol. 2007. PMID: 17665183 Clinical Trial.
-
Clinical pharmacokinetics and summary of efficacy and tolerability of atazanavir.Clin Pharmacokinet. 2005;44(10):1035-50. doi: 10.2165/00003088-200544100-00003. Clin Pharmacokinet. 2005. PMID: 16176117 Review.
-
Atazanavir/ritonavir: a review of its use in HIV therapy.Drugs Today (Barc). 2008 Feb;44(2):103-32. doi: 10.1358/dot.2008.44.2.1137107. Drugs Today (Barc). 2008. PMID: 18389089 Review.
-
Effect of topiramate on the pharmacokinetics of an oral contraceptive containing norethindrone and ethinyl estradiol in patients with epilepsy.Epilepsia. 1997 Mar;38(3):317-23. doi: 10.1111/j.1528-1157.1997.tb01123.x. Epilepsia. 1997. PMID: 9070594
Cited by
-
Hormonal contraceptive use in HIV-infected women using antiretroviral therapy: A Systematic review.Open Access J Contracept. 2015;6:37-520. doi: 10.2147/OAJC.S55038. Epub 2015 May 7. Open Access J Contracept. 2015. PMID: 28955156 Free PMC article.
-
Interactions between Hormonal Contraception and Anti-Retroviral Therapy: An Updated Review.Curr Obstet Gynecol Rep. 2020 Sep;9(3):98-104. doi: 10.1007/s13669-020-00289-7. Epub 2020 May 31. Curr Obstet Gynecol Rep. 2020. PMID: 33552676 Free PMC article.
-
Pharmacokinetics of levonorgestrel and etonogestrel contraceptive implants over 48 weeks with rilpivirine- or darunavir-based antiretroviral therapy.J Antimicrob Chemother. 2022 Oct 28;77(11):3144-3152. doi: 10.1093/jac/dkac296. J Antimicrob Chemother. 2022. PMID: 36059130 Free PMC article.
-
Society of Family Planning Clinical Recommendations: Contraceptive Care in the Context of Pandemic Response.Contraception. 2022 Sep;113:1-12. doi: 10.1016/j.contraception.2022.05.006. Epub 2022 May 18. Contraception. 2022. PMID: 35594989 Free PMC article.
-
Current and future contraceptive options for women living with HIV.Expert Opin Pharmacother. 2018 Jan;19(1):1-12. doi: 10.1080/14656566.2017.1378345. Epub 2017 Sep 19. Expert Opin Pharmacother. 2018. PMID: 28891343 Free PMC article. Review.
References
-
- World Health Organization . Women and health: today's evidence tomorrow's agenda. Geneva, Switzerland: 2009. (2009)
-
- PMTCT strategic vision 2010-2015. Preventing mother-to-child transmission of HIV to reach the UNGASS and Millennium Development Goals World Health Organization. WHO Press; Switzerland: Feb 2, 2010.
-
- Dresser GK, Spence JD, Bailey DG. Pharmacokinetic-pharmacodynamic consequences and clinical relevance of cytochrome P450 3A4 inhibition. Clin Pharmacokinet. 2000;38:41–57. - PubMed
-
- Zeldin RK, Petruschke RA. Pharmacological and therapeutic properties of ritonavir-boosted protease inhibitor therapy in HIV-infected patients. J Antimicrob Chemother. 2004;53:4–9. - PubMed
-
- Korhonen T, Turpeinen M, Tolonen A, Laine K, Pelkonen O. Identification of the human cytochrome P450 enzymes involved in the in vitro biotransformation of lynestrenol and norethindrone. J Steroid Biochem Mol Biol. 2008;110:56–66. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

