A Phase II trial of a combination herbal supplement for men with biochemically recurrent prostate cancer
- PMID: 25245366
- PMCID: PMC4234307
- DOI: 10.1038/pcan.2014.37
A Phase II trial of a combination herbal supplement for men with biochemically recurrent prostate cancer
Abstract
Background: Men with biochemical recurrence (BCR) of prostate cancer are typically observed or treated with androgen-deprivation therapy. Non-hormonal, non-toxic treatments to slow the rise of PSA are desirable. We studied a combination herbal supplement, Prostate Health Cocktail (PHC), in prostate cancer cell lines and in a population of men with BCR.
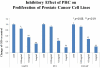
Methods: PC3, LAPC3 and LNCaP cells were incubated with increasing concentrations of PHC suspension. Men previously treated for prostate cancer with surgery, radiation or both with rising PSA but no radiographic metastases were treated with three capsules of PHC daily; the primary end point was 50% PSA decline. Circulating tumor cells (CTCs) were identified using parylene membrane filters.
Results: PHC showed a strong dose-dependent anti-proliferative effect in androgen-sensitive and independent cell lines in vitro and suppression of androgen receptor expression. Forty eligible patients were enrolled in the clinical trial. Median baseline PSA was 2.8 ng ml(-1) (1.1-84.1) and 15 men (38%) had a PSA decline on study (1-55% reduction); 25 (62%) had rising PSA on study. The median duration of PSA stability was 6.4 months. Two patients had grade 2/3 transaminitis; the only other grade 2 toxicities were hyperglycemia, hypercalcemia and flatulence. There were no significant changes in testosterone or dihydrotestosterone. CTCs were identified in 19 men (47%).
Conclusions: Although the primary end point was not met, PHC was well tolerated and was associated with PSA declines and stabilization in a significant number of patients. We believe this is the first report of detecting CTCs in men with BCR prostate cancer. Randomized studies are needed to better define the effect of PHC in men with BCR.
Figures





References
-
- Keating NL, O'Malley J, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J Clin Oncol. 2008;24:4448–56. - PubMed
-
- Ross RW, Small EJ. Osteoporosis in men treated with androgen deprivation therapy for prostate cancer. J Urol. 2002;167:1952. - PubMed
-
- Fowler FJ, Jr, Collins MM, Corkery EW, Elliott DB, Barry MJ. The impact of androgen deprivation on quality of life after radical prostatectomy for prostate carcinoma. Cancer. 2002;95:287–295. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

