Transcription factors bind negatively selected sites within human mtDNA genes
- PMID: 25245407
- PMCID: PMC4224337
- DOI: 10.1093/gbe/evu210
Transcription factors bind negatively selected sites within human mtDNA genes
Abstract
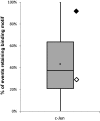
Transcription of mitochondrial DNA (mtDNA)-encoded genes is thought to be regulated by a handful of dedicated transcription factors (TFs), suggesting that mtDNA genes are separately regulated from the nucleus. However, several TFs, with known nuclear activities, were found to bind mtDNA and regulate mitochondrial transcription. Additionally, mtDNA transcriptional regulatory elements, which were proved important in vitro, were harbored by a deletion that normally segregated among healthy individuals. Hence, mtDNA transcriptional regulation is more complex than once thought. Here, by analyzing ENCODE chromatin immunoprecipitation sequencing (ChIP-seq) data, we identified strong binding sites of three bona fide nuclear TFs (c-Jun, Jun-D, and CEBPb) within human mtDNA protein-coding genes. We validated the binding of two TFs by ChIP-quantitative polymerase chain reaction (c-Jun and Jun-D) and showed their mitochondrial localization by electron microscopy and subcellular fractionation. As a step toward investigating the functionality of these TF-binding sites (TFBS), we assessed signatures of selection. By analyzing 9,868 human mtDNA sequences encompassing all major global populations, we recorded genetic variants in tips and nodes of mtDNA phylogeny within the TFBS. We next calculated the effects of variants on binding motif prediction scores. Finally, the mtDNA variation pattern in predicted TFBS, occurring within ChIP-seq negative-binding sites, was compared with ChIP-seq positive-TFBS (CPR). Motifs within CPRs of c-Jun, Jun-D, and CEBPb harbored either only tip variants or their nodal variants retained high motif prediction scores. This reflects negative selection within mtDNA CPRs, thus supporting their functionality. Hence, human mtDNA-coding sequences may have dual roles, namely coding for genes yet possibly also possessing regulatory potential.
Keywords: CEBPb; ChIP-seq; Jun-D; c-Jun; mitochondrial DNA; negative selection; transcription.
© The Author(s) 2014. Published by Oxford University Press on behalf of the Society for Molecular Biology and Evolution.
Figures




Similar articles
-
Role of ChIP-seq in the discovery of transcription factor binding sites, differential gene regulation mechanism, epigenetic marks and beyond.Cell Cycle. 2014;13(18):2847-52. doi: 10.4161/15384101.2014.949201. Cell Cycle. 2014. PMID: 25486472 Free PMC article. Review.
-
Improving analysis of transcription factor binding sites within ChIP-Seq data based on topological motif enrichment.BMC Genomics. 2014 Jun 13;15(1):472. doi: 10.1186/1471-2164-15-472. BMC Genomics. 2014. PMID: 24927817 Free PMC article.
-
Contribution of nucleosome binding preferences and co-occurring DNA sequences to transcription factor binding.BMC Genomics. 2013 Jun 28;14:428. doi: 10.1186/1471-2164-14-428. BMC Genomics. 2013. PMID: 23805837 Free PMC article.
-
Transcription factor-binding k-mer analysis clarifies the cell type dependency of binding specificities and cis-regulatory SNPs in humans.BMC Genomics. 2023 Oct 7;24(1):597. doi: 10.1186/s12864-023-09692-9. BMC Genomics. 2023. PMID: 37805453 Free PMC article.
-
An algorithmic perspective of de novo cis-regulatory motif finding based on ChIP-seq data.Brief Bioinform. 2018 Sep 28;19(5):1069-1081. doi: 10.1093/bib/bbx026. Brief Bioinform. 2018. PMID: 28334268 Review.
Cited by
-
Higher Order Organization of the mtDNA: Beyond Mitochondrial Transcription Factor A.Front Genet. 2019 Dec 20;10:1285. doi: 10.3389/fgene.2019.01285. eCollection 2019. Front Genet. 2019. PMID: 31998357 Free PMC article. Review.
-
Exonic splice regulation imposes strong selection at synonymous sites.Genome Res. 2018 Oct;28(10):1442-1454. doi: 10.1101/gr.233999.117. Epub 2018 Aug 24. Genome Res. 2018. PMID: 30143596 Free PMC article.
-
Ancient Out-of-Africa Mitochondrial DNA Variants Associate with Distinct Mitochondrial Gene Expression Patterns.PLoS Genet. 2016 Nov 3;12(11):e1006407. doi: 10.1371/journal.pgen.1006407. eCollection 2016 Nov. PLoS Genet. 2016. PMID: 27812116 Free PMC article.
-
Mapping and editing animal mitochondrial genomes: can we overcome the challenges?Philos Trans R Soc Lond B Biol Sci. 2020 Jan 20;375(1790):20190187. doi: 10.1098/rstb.2019.0187. Epub 2019 Dec 2. Philos Trans R Soc Lond B Biol Sci. 2020. PMID: 31787046 Free PMC article. Review.
-
mtDNA Chromatin-like Organization Is Gradually Established during Mammalian Embryogenesis.iScience. 2019 Feb 22;12:141-151. doi: 10.1016/j.isci.2018.12.032. Epub 2019 Jan 8. iScience. 2019. PMID: 30684873 Free PMC article.
References
-
- Andrews RM, et al. Reanalysis and revision of the Cambridge reference sequence for human mitochondrial DNA. Nat Genet. 1999;23:147. - PubMed
-
- Bar-Yaacov D, Blumberg A, Mishmar D. Mitochondrial-nuclear co-evolution and its effects on OXPHOS activity and regulation. Biochim Biophys Acta. 2012;1819:1107–1111. - PubMed
-
- Bi R, et al. The acquisition of an inheritable 50-bp deletion in the human mtDNA control region does not affect the mtDNA copy number in peripheral blood cells. Hum Mutat. 2010;31:538–543. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

