Nuclear respiratory factor 2 regulates the transcription of AMPA receptor subunit GluA2 (Gria2)
- PMID: 25245478
- PMCID: PMC4198421
- DOI: 10.1016/j.bbamcr.2014.09.006
Nuclear respiratory factor 2 regulates the transcription of AMPA receptor subunit GluA2 (Gria2)
Abstract
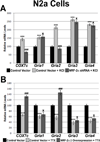
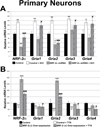
Neuronal activity is highly dependent on energy metabolism. Nuclear respiratory factor 2 (NRF-2) tightly couples neuronal activity and energy metabolism by transcriptionally co-regulating all 13 subunits of an important energy-generating enzyme, cytochrome c oxidase (COX), as well as critical subunits of excitatory NMDA receptors. AMPA receptors are another major class of excitatory glutamatergic receptors that mediate most of the fast excitatory synaptic transmission in the brain. They are heterotetrameric proteins composed of various combinations of GluA1-4 subunits, with GluA2 being the most common one. We have previously shown that GluA2 (Gria2) is transcriptionally regulated by nuclear respiratory factor 1 (NRF-1) and specificity protein 4 (Sp4), which also regulate all subunits of COX. However, it was not known if NRF-2 also couples neuronal activity and energy metabolism by regulating subunits of the AMPA receptors. By means of multiple approaches, including electrophoretic mobility shift and supershift assays, chromatin immunoprecipitation, promoter mutations, real-time quantitative PCR, and western blot analysis, NRF-2 was found to functionally regulate the expression of Gria2, but not of Gria1, Gria3, or Gria4 genes in neurons. By regulating the GluA2 subunit of the AMPA receptor, NRF-2 couples energy metabolism and neuronal activity at the transcriptional level through a concurrent and parallel mechanism with NRF-1 and Sp4.
Keywords: AMPA receptor; GABP; Gene regulation; GluA2; Nuclear respiratory factor 2; Transcription factor.
Copyright © 2014 Elsevier B.V. All rights reserved.
Conflict of interest statement
Conflict of Interest: The authors declare no competing financial interest.
Figures





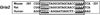
Similar articles
-
Specificity protein 4 (Sp4) regulates the transcription of AMPA receptor subunit GluA2 (Gria2).Biochim Biophys Acta. 2014 Jun;1843(6):1196-206. doi: 10.1016/j.bbamcr.2014.02.008. Epub 2014 Feb 24. Biochim Biophys Acta. 2014. PMID: 24576410 Free PMC article.
-
Nuclear respiratory factor 2 regulates the expression of the same NMDA receptor subunit genes as NRF-1: both factors act by a concurrent and parallel mechanism to couple energy metabolism and synaptic transmission.Biochim Biophys Acta. 2013 Jan;1833(1):48-58. doi: 10.1016/j.bbamcr.2012.10.014. Epub 2012 Oct 23. Biochim Biophys Acta. 2013. PMID: 23085505 Free PMC article.
-
Nuclear respiratory factor 1 co-regulates AMPA glutamate receptor subunit 2 and cytochrome c oxidase: tight coupling of glutamatergic transmission and energy metabolism in neurons.J Neurochem. 2009 Mar;108(6):1595-606. doi: 10.1111/j.1471-4159.2009.05929.x. Epub 2009 Jan 23. J Neurochem. 2009. PMID: 19166514 Free PMC article.
-
Regulation of Na(+)/K(+)-ATPase by nuclear respiratory factor 1: implication in the tight coupling of neuronal activity, energy generation, and energy consumption.J Biol Chem. 2012 Nov 23;287(48):40381-90. doi: 10.1074/jbc.M112.414573. Epub 2012 Oct 9. J Biol Chem. 2012. PMID: 23048038 Free PMC article.
-
Bigenomic regulation of cytochrome c oxidase in neurons and the tight coupling between neuronal activity and energy metabolism.Adv Exp Med Biol. 2012;748:283-304. doi: 10.1007/978-1-4614-3573-0_12. Adv Exp Med Biol. 2012. PMID: 22729863 Free PMC article. Review.
Cited by
-
Neuroprotective and antimalarial effects of Juglans regia leaf extracts in a murine model of cerebral malaria.Front Vet Sci. 2025 Apr 7;12:1537686. doi: 10.3389/fvets.2025.1537686. eCollection 2025. Front Vet Sci. 2025. PMID: 40260212 Free PMC article.
-
Transcriptional Regulation of Brain-derived Neurotrophic Factor Coding Exon IX: ROLE OF NUCLEAR RESPIRATORY FACTOR 2.J Biol Chem. 2016 Oct 21;291(43):22583-22593. doi: 10.1074/jbc.M116.742304. Epub 2016 Sep 13. J Biol Chem. 2016. PMID: 27624937 Free PMC article.
-
Specificity protein 4 (Sp4) transcriptionally regulates inhibitory GABAergic receptors in neurons.Biochim Biophys Acta. 2016 Jan;1863(1):1-9. doi: 10.1016/j.bbamcr.2015.10.005. Epub 2015 Oct 18. Biochim Biophys Acta. 2016. PMID: 26469128 Free PMC article.
-
Effects of nuclear respiratory factor‑1 on apoptosis and mitochondrial dysfunction induced by cobalt chloride in H9C2 cells.Mol Med Rep. 2019 Mar;19(3):2153-2163. doi: 10.3892/mmr.2019.9839. Epub 2019 Jan 10. Mol Med Rep. 2019. PMID: 30628711 Free PMC article.
-
Knockdown of GluA2 induces apoptosis in non-small-cell lung cancer A549 cells through the p53 signaling pathway.Oncol Lett. 2017 Jul;14(1):1005-1010. doi: 10.3892/ol.2017.6234. Epub 2017 May 24. Oncol Lett. 2017. PMID: 28693266 Free PMC article.
References
-
- Isaac JT, Ashby MC, McBain CJ. The role of the GluR2 subunit in AMPA receptor function and synaptic plasticity. Neuron. 2007;54:859–871. - PubMed
-
- Malinow R, Malenka RC. AMPA receptor trafficking and synaptic plasticity. Annu Rev Neurosci. 2002;25:103–126. - PubMed
-
- Shepherd JD, Huganir RL. The cell biology of synaptic plasticity: AMPA receptor trafficking. Annu Rev Cell Dev Biol. 2007;23:613–643. - PubMed
-
- Borges K, Dingledine R. AMPA receptors: molecular and functional diversity. Prog Brain Res. 1998;116:153–170. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

