α1-antitrypsin modulates microglial-mediated neuroinflammation and protects microglial cells from amyloid-β-induced toxicity
- PMID: 25245568
- PMCID: PMC4177587
- DOI: 10.1186/s12974-014-0165-8
α1-antitrypsin modulates microglial-mediated neuroinflammation and protects microglial cells from amyloid-β-induced toxicity
Abstract
Background: One hallmark of Alzheimer disease is microglial activation. Therapeutic approaches for this neurodegenerative disease include the modulation of microglial cells. α1-antitrypsin (A1AT) has been shown to exert anti-inflammatory effects on macrophages and lung epithelial cells and an inhibition of calpain activity in neutrophil granulocytes. Nothing is known about the effect of A1AT on microglial-mediated neuroinflammation. Our aim was to investigate the effect of A1AT on amyloid-β (Aβ)- and LPS-treated microglial cells in vitro with respect to cytokine production, stress pathways, cell viability, phagocytotic abilities and the underlying mechanisms.
Methods: Primary microglial cells were isolated from Swiss Webster mouse embryos on embryonic day 13.5. Cytokines in the supernatants of treated primary microglial cells were analyzed with ELISAs, and accumulated nitrite was detected with Griess reagents. Intracellular stress pathways were investigated in cell lysates using western blotting. Intracellular calcium levels were detected in BV-2 microglial cells loaded with the Ca2+-sensitive (fluorescent) dye Fluo-4. Calpain activity in primary microglial cells was assessed by using a calpain activity assay. Cell viability of Aβ-treated microglial cells was analyzed using MTT assay. Phagocytosis of Aβ was evaluated with western blot analysis.
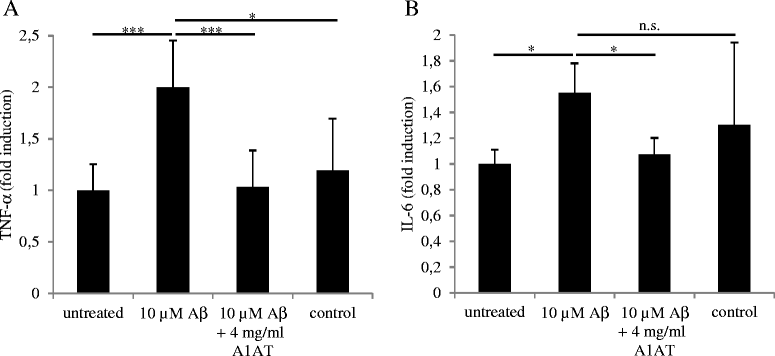
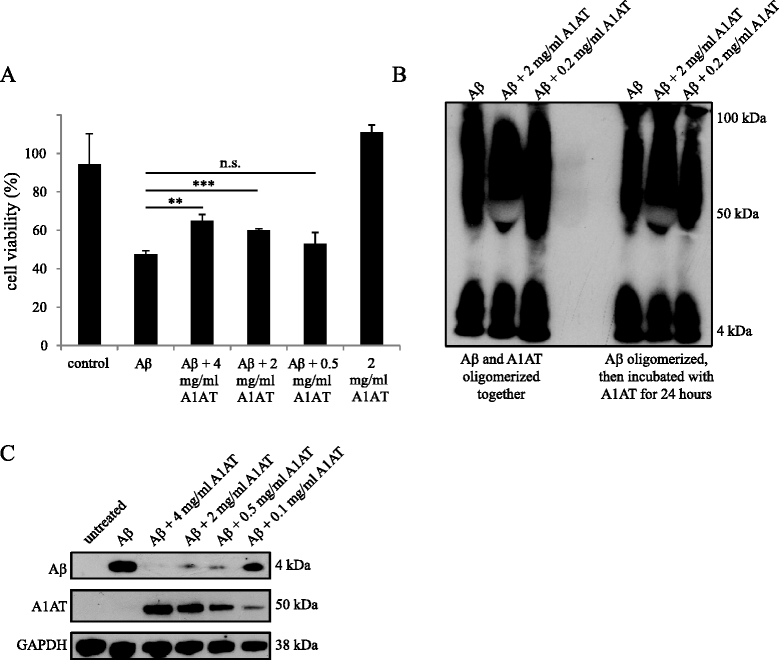
Results: Upon co-administration, A1AT reduced pro-inflammatory mediators induced by LPS or Aβ. Interestingly, we detected a reduction in calpain activity and in the concentration of intracellular calcium that might mediate the anti-inflammatory effects of A1AT. Inhibition of the classic activation pathways, such as phosphorylation of mitogen-activated protein kinases or activation of protein kinase A were excluded as a mechanism of A1AT-mediated effects. In addition, A1AT increased the viability of Aβ-treated microglial cells and reduced Aβ phagocytosis.
Conclusions: We provide evidence on the mechanism of action of A1AT on microglial-mediated neuroinflammation in vitro. Our in vitro data indicate that A1AT treatment modulates microglial cells in inflammatory conditions and that this modulation is due to an inhibition of calpain activity and intracellular calcium levels. The underlying mechanisms of the effects observed here are promising for future therapeutic strategies and should thus be further pursued in transgenic mouse models of Alzheimer disease.
Figures



References
-
- Cho S, Kim Y, Cruz MO, Park EM, Chu CK, Song GY, Joh TH. Repression of proinflammatory cytokine and inducible nitric oxide synthase (NOS2) gene expression in activated microglia by N-acetyl-O-methyldopamine: protein kinase A-dependent mechanism. Glia. 2001;33:324–333. doi: 10.1002/1098-1136(20010315)33:4<324::AID-GLIA1031>3.0.CO;2-M. - DOI - PubMed
-
- Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Eikelenboom P, Emmerling M, Fiebich BL, Finch CE, Frautschy S, Griffin WS, Hampel H, Hull M, Landreth G, Lue L, Mrak R, Mackenzie IR, McGeer PL, O'Banion MK, Pachter J, Pasinetti G, Plata-Salaman C, Rogers J, Rydel R, Shen Y, Streit W, Strohmeyer R, Tooyoma I, et al. Inflammation and Alzheimer's disease. Neurobiol Aging. 2000;21:383–421. doi: 10.1016/S0197-4580(00)00124-X. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

