3D culture assays of murine mammary branching morphogenesis and epithelial invasion
- PMID: 25245692
- PMCID: PMC4750493
- DOI: 10.1007/978-1-4939-1164-6_10
3D culture assays of murine mammary branching morphogenesis and epithelial invasion
Abstract
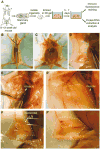
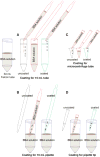
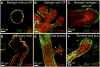
Epithelia are fundamental tissues that line cavities, glands, and outer body surfaces. We use three-dimensional (3D) embedded culture of primary murine mammary epithelial ducts, called "organoids," to recapitulate in days in culture epithelial programs that occur over weeks deep within the body. Modulating the composition of the extracellular matrix (ECM) allows us to model cell- and tissue-level behaviors observed in normal development, such as branching morphogenesis, and in cancer, such as invasion and dissemination. Here, we describe a collection of protocols for 3D culture of mammary organoids in different ECMs and for immunofluorescence staining of 3D culture samples and mammary gland tissue sections. We illustrate expected phenotypic outcomes of each assay and provide troubleshooting tips for commonly encountered technical problems.
Figures





Similar articles
-
Mammary collective cell migration involves transient loss of epithelial features and individual cell migration within the epithelium.J Cell Sci. 2012 Jun 1;125(Pt 11):2638-54. doi: 10.1242/jcs.096875. Epub 2012 Feb 17. J Cell Sci. 2012. PMID: 22344263 Free PMC article.
-
Constructing three-dimensional models to study mammary gland branching morphogenesis and functional differentiation.J Mammary Gland Biol Neoplasia. 2012 Jun;17(2):103-10. doi: 10.1007/s10911-012-9251-7. Epub 2012 May 10. J Mammary Gland Biol Neoplasia. 2012. PMID: 22573197 Review.
-
Optimising a self-assembling peptide hydrogel as a Matrigel alternative for 3-dimensional mammary epithelial cell culture.Biomater Adv. 2024 Jun;160:213847. doi: 10.1016/j.bioadv.2024.213847. Epub 2024 Mar 28. Biomater Adv. 2024. PMID: 38657288
-
Three-dimensional cultures of mouse mammary epithelial cells.Methods Mol Biol. 2013;945:221-50. doi: 10.1007/978-1-62703-125-7_14. Methods Mol Biol. 2013. PMID: 23097110 Free PMC article.
-
Mammary gland 3D cell culture systems in farm animals.Vet Res. 2021 Jun 2;52(1):78. doi: 10.1186/s13567-021-00947-5. Vet Res. 2021. PMID: 34078471 Free PMC article. Review.
Cited by
-
3D culture models for studying branching morphogenesis in the mammary gland and mammalian lung.Biomaterials. 2019 Apr;198:135-145. doi: 10.1016/j.biomaterials.2018.08.043. Epub 2018 Aug 23. Biomaterials. 2019. PMID: 30174198 Free PMC article. Review.
-
E-cadherin is required for metastasis in multiple models of breast cancer.Nature. 2019 Sep;573(7774):439-444. doi: 10.1038/s41586-019-1526-3. Epub 2019 Sep 4. Nature. 2019. PMID: 31485072 Free PMC article.
-
Multiple roles for Bcl-3 in mammary gland branching, stromal collagen invasion, involution and tumor pathology.Breast Cancer Res. 2022 Jun 9;24(1):40. doi: 10.1186/s13058-022-01536-w. Breast Cancer Res. 2022. PMID: 35681213 Free PMC article.
-
Sfrp3 modulates stromal-epithelial crosstalk during mammary gland development by regulating Wnt levels.Nat Commun. 2019 Jun 6;10(1):2481. doi: 10.1038/s41467-019-10509-1. Nat Commun. 2019. PMID: 31171792 Free PMC article.
-
Using Biosensors to Study Organoids, Spheroids and Organs-on-a-Chip: A Mechanobiology Perspective.Biosensors (Basel). 2023 Sep 24;13(10):905. doi: 10.3390/bios13100905. Biosensors (Basel). 2023. PMID: 37887098 Free PMC article. Review.
References
-
- Hogg NA, Harrison CJ, Tickle C. Lumen formation in the developing mouse mammary gland. J Embryol Exp Morphol. 1983;73:39–57. - PubMed
-
- Williams JM, Daniel CW. Mammary ductal elongation: differentiation of myoepithelium and basal lamina during branching morphogenesis. Dev Biol. 1983;97:274–290. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

