Activation of calcineurin underlies altered trafficking of α2 subunit containing GABAA receptors during prolonged epileptiform activity
- PMID: 25245802
- PMCID: PMC4239296
- DOI: 10.1016/j.neuropharm.2014.09.014
Activation of calcineurin underlies altered trafficking of α2 subunit containing GABAA receptors during prolonged epileptiform activity
Abstract
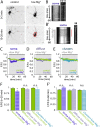
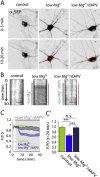
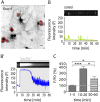
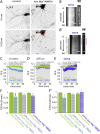
Fast inhibitory signalling in the mammalian brain is mediated by gamma-aminobutyric acid type A receptors (GABAARs), which are targets for anti-epileptic therapy such as benzodiazepines. GABAARs undergo tightly regulated trafficking processes that are essential for maintenance and physiological modulation of inhibitory strength. The trafficking of GABAARs to and from the membrane is altered during prolonged seizures such as in Status Epilepticus (SE) and has been suggested to contribute to benzodiazepine pharmacoresistance in patients with SE. However, the intracellular signalling mechanisms that cause this modification in GABAAR trafficking remain poorly understood. In this study, we investigate the surface stability of GABAARs during SE utilising the low Mg(2+) model in hippocampal rat neurons. Live-cell imaging of super ecliptic pHluorin (SEP)-tagged α2 subunit containing GABAARs during low Mg(2+) conditions reveals that the somatic surface receptor pool undergoes down-regulation dependent on N-methyl-d-aspartate receptor (NMDAR) activity. Analysis of the intracellular Ca(2+) signal during low Mg(2+) using the Ca(2+)-indicator Fluo4 shows that this reduction of surface GABAARs correlates well with the timeline of intracellular Ca(2+) changes. Furthermore, we show that the activation of the phosphatase calcineurin was required for the decrease in surface GABAARs in neurons undergoing epileptiform activity. These results indicate that somatic modulation of GABAAR trafficking during epileptiform activity in vitro is mediated by calcineurin activation which is linked to changes in intracellular Ca(2+) concentrations. These mechanisms could account for benzodiazepine pharmacoresistance and the maintenance of recurrent seizure activity, and reveal potential novel targets for the treatment of SE.
Keywords: Calcium signaling; Epilepsy; GABA(A) receptor; Surface stability; Trafficking.
Copyright © 2014 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures
References
-
- Albowitz B., König P., Kuhnt U. Spatiotemporal distribution of intracellular calcium transients during epileptiform activity in guinea pig hippocampal slices. J. Neurophysiol. 1997;77(1):491–501. - PubMed
-
- Bannai H. Activity-dependent tuning of inhibitory neurotransmission based on GABAAR diffusion dynamics. Neuron. 2009;62(5):670–682. - PubMed
-
- Blair R.E. Epileptogenesis causes acute and chronic increases in GABAA receptor endocytosis that contributes to the induction and maintenance of seizures in the hippocampal culture model of acquired epilepsy. J. Pharmacol. Exp. Ther. 2004;310(3):871–880. http://www.ncbi.nlm.nih.gov/pubmed/15084648 Available at: (accessed 11.06.14.) - PubMed
-
- Bogdanov Y. Synaptic GABAA receptors are directly recruited from their extrasynaptic counterparts. EMBO J. 2006;25(18):4381–4389. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1570424&tool=p... Available at: (accessed 28.05.14.) - PMC - PubMed
-
- Brünig I. Intact sorting, targeting, and clustering of gamma-aminobutyric acid A receptor subtypes in hippocampal neurons in vitro. J. Comp. Neurol. 2002;443(1):43–55. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

