Antibodies to Staphylococcus aureus serotype 8 capsular polysaccharide react with and protect against serotype 5 and 8 isolates
- PMID: 25245803
- PMCID: PMC4249259
- DOI: 10.1128/IAI.02373-14
Antibodies to Staphylococcus aureus serotype 8 capsular polysaccharide react with and protect against serotype 5 and 8 isolates
Abstract
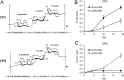
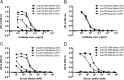
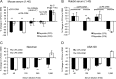
Most Staphylococcus aureus isolates produce either a serotype 5 (CP5) or 8 (CP8) capsular polysaccharide, and the CP antigens are targets for vaccine development. Since CP5 and CP8 have similar trisaccharide repeating units, it is important to identify an epitope shared by both CP5 and CP8. To characterize cross-reactivity between CP5 and CP8, the immunogenicity of CP5 and CP8 conjugate vaccines in mice and rabbits was evaluated by serological assays. Immune sera were also tested for functional activity by in vitro opsonophagocytic-killing assays and a murine bacteremia model. Antibodies to the CP5-cross-reactive material 197 (CRM197) conjugate vaccine bound only to purified CP5. In contrast, antibodies to the CP8-CRM conjugate vaccine reacted with CP8 and (to a lesser extent) CP5. De-O-acetylation of CP5 increased its reactivity with CP8 antibodies. Moreover, CP8 antibodies bound to Pseudomonas aeruginosa O11 lipopolysaccharide, which has a trisaccharide repeating unit similar to that of the S. aureus CPs. CP8-CRM antibodies mediated in vitro opsonophagocytic killing of S. aureus expressing CP5 or CP8, whereas CP5-CRM antibodies were serotype specific. Passive immunization with antiserum to CP5-CRM or CP8-CRM protected mice against bacteremia induced by a serotype 5 S. aureus isolate, suggesting that CP8-CRM elicits antibodies cross-reactive to CP5. The identification of epitopes shared by CP5 and CP8 may inform the rational design of a vaccine to protect against infections caused by CP5- or CP8-producing strains of S. aureus.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures




Similar articles
-
Evaluation of serotypes 5 and 8 capsular polysaccharides in protection against Staphylococcus aureus in murine models of infection.Hum Vaccin Immunother. 2017 Jul 3;13(7):1609-1614. doi: 10.1080/21645515.2017.1304334. Epub 2017 Apr 19. Hum Vaccin Immunother. 2017. PMID: 28422567 Free PMC article.
-
Antibodies to Staphylococcus aureus capsular polysaccharides 5 and 8 perform similarly in vitro but are functionally distinct in vivo.Virulence. 2017 Aug 18;8(6):859-874. doi: 10.1080/21505594.2016.1270494. Epub 2016 Dec 9. Virulence. 2017. PMID: 27936346 Free PMC article.
-
Safety, tolerability, and immunogenicity of a single dose 4-antigen or 3-antigen Staphylococcus aureus vaccine in healthy older adults: Results of a randomised trial.Vaccine. 2017 Jan 5;35(2):385-394. doi: 10.1016/j.vaccine.2016.11.032. Epub 2016 Nov 17. Vaccine. 2017. PMID: 27866765 Clinical Trial.
-
Pseudomonas aeruginosa antigens as potential vaccines.FEMS Microbiol Rev. 1997 Nov;21(3):243-77. doi: 10.1111/j.1574-6976.1997.tb00353.x. FEMS Microbiol Rev. 1997. PMID: 9451816 Review.
-
Use of a conjugate polysaccharide vaccine in the prevention of invasive staphylococcal disease: is an additional vaccine needed or possible?Vaccine. 2006 Apr 12;24 Suppl 2:S2-65-9. doi: 10.1016/j.vaccine.2005.01.126. Vaccine. 2006. PMID: 16823932 Review.
Cited by
-
Evaluation of serotypes 5 and 8 capsular polysaccharides in protection against Staphylococcus aureus in murine models of infection.Hum Vaccin Immunother. 2017 Jul 3;13(7):1609-1614. doi: 10.1080/21645515.2017.1304334. Epub 2017 Apr 19. Hum Vaccin Immunother. 2017. PMID: 28422567 Free PMC article.
-
Investigational drugs to treat methicillin-resistant Staphylococcus aureus.Expert Opin Investig Drugs. 2016;25(1):73-93. doi: 10.1517/13543784.2016.1109077. Epub 2015 Nov 4. Expert Opin Investig Drugs. 2016. PMID: 26536498 Free PMC article. Review.
-
Detection of capsular genotypes of methicillin-resistant Staphylococcus aureus and clonal distribution of the cap5 and cap8 genes in clinical isolates.Arch Microbiol. 2022 Feb 21;204(3):186. doi: 10.1007/s00203-022-02793-1. Arch Microbiol. 2022. PMID: 35192046 Free PMC article.
-
Exploiting dominant-negative toxins to combat Staphylococcus aureus pathogenesis.EMBO Rep. 2016 Mar;17(3):428-40. doi: 10.15252/embr.201540994. Epub 2016 Feb 8. EMBO Rep. 2016. PMID: 26882549 Free PMC article.
-
Acapsular Staphylococcus aureus with a non-functional agr regains capsule expression after passage through the bloodstream in a bacteremia mouse model.Sci Rep. 2020 Aug 24;10(1):14108. doi: 10.1038/s41598-020-70671-1. Sci Rep. 2020. PMID: 32839485 Free PMC article.
References
-
- Hochkeppel HK, Braun DG, Vischer W, Imm A, Sutter S, Staeubli U, Guggenheim R, Kaplan EL, Boutonnier A, Fournier JM. 1987. Serotyping and electron microscopy studies of Staphylococcus aureus clinical isolates with monoclonal antibodies to capsular polysaccharide types 5 and 8. J. Clin. Microbiol. 25:526–530. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

