Human endogenous retroviral elements promote genome instability via non-allelic homologous recombination
- PMID: 25246103
- PMCID: PMC4195946
- DOI: 10.1186/s12915-014-0074-4
Human endogenous retroviral elements promote genome instability via non-allelic homologous recombination
Abstract
Background: Recurrent rearrangements of the human genome resulting in disease or variation are mainly mediated by non-allelic homologous recombination (NAHR) between low-copy repeats. However, other genomic structures, including AT-rich palindromes and retroviruses, have also been reported to underlie recurrent structural rearrangements. Notably, recurrent deletions of Yq12 conveying azoospermia, as well as non-pathogenic reciprocal duplications, are mediated by human endogenous retroviral elements (HERVs). We hypothesized that HERV elements throughout the genome can serve as substrates for genomic instability and result in human copy-number variation (CNV).
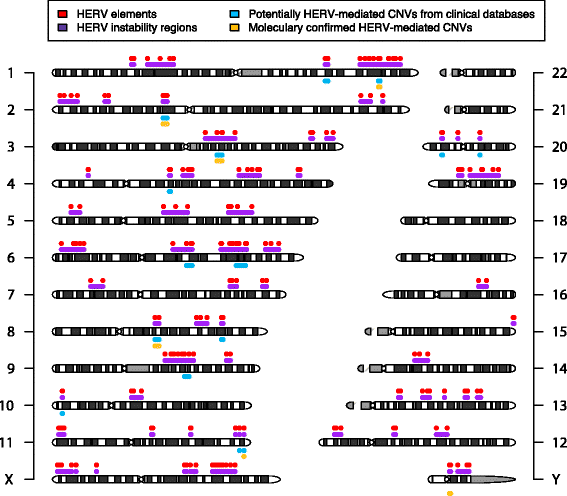
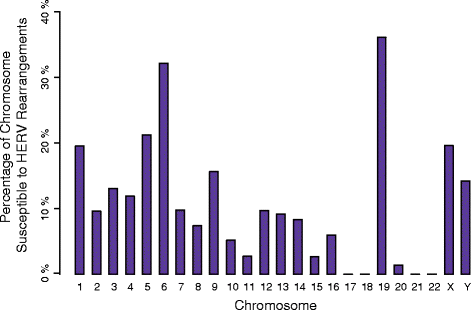
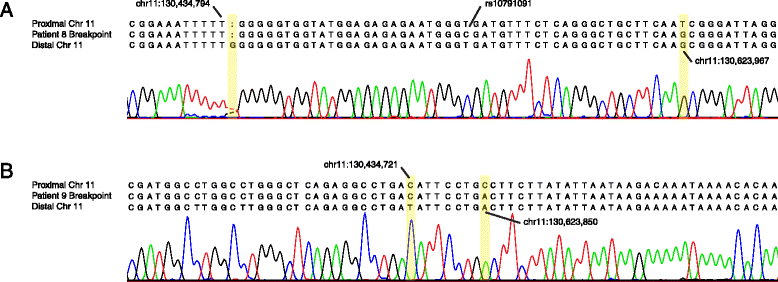
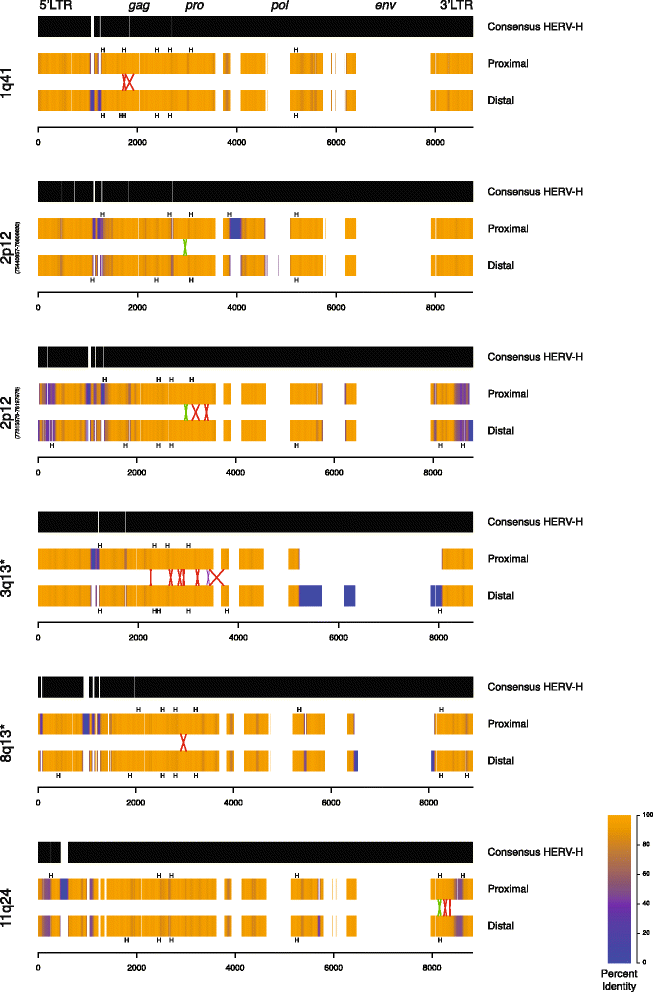
Results: We developed parameters to identify HERV elements similar to those that mediate Yq12 rearrangements as well as recurrent deletions of 3q13.2q13.31. We used these parameters to identify HERV pairs genome-wide that may cause instability. Our analysis highlighted 170 pairs, flanking 12.1% of the genome. We cross-referenced these predicted susceptibility regions with CNVs from our clinical databases for potentially HERV-mediated rearrangements and identified 78 CNVs. We subsequently molecularly confirmed recurrent deletion and duplication rearrangements at four loci in ten individuals, including reciprocal rearrangements at two loci. Breakpoint sequencing revealed clustering in regions of high sequence identity enriched in PRDM9-mediated recombination hotspot motifs.
Conclusions: The presence of deletions and reciprocal duplications suggests NAHR as the causative mechanism of HERV-mediated CNV, even though the length and the sequence homology of the HERV elements are less than currently thought to be required for NAHR. We propose that in addition to HERVs, other repetitive elements, such as long interspersed elements, may also be responsible for the formation of recurrent CNVs via NAHR.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

