Angiotensin-(1-7) abolishes AGE-induced cellular hypertrophy and myofibroblast transformation via inhibition of ERK1/2
- PMID: 25246357
- PMCID: PMC4254268
- DOI: 10.1016/j.cellsig.2014.09.010
Angiotensin-(1-7) abolishes AGE-induced cellular hypertrophy and myofibroblast transformation via inhibition of ERK1/2
Abstract
Angiotensin-(1-7) (Ang-(1-7))/AT7-Mas receptor axis is an alternative pathway within the renin-angiotensin system (RAS) that generally opposes the actions of Ang II/AT1 receptor pathway. Advanced glycated end product (AGEs) including glucose- and methylglyoxal-modified albumin (MGA) may contribute to the development and progression of diabetic nephropathy in part through activation of the Ang II/AT1 receptor system; however, the influence of AGE on the Ang-(1-7) arm of the RAS within the kidney is unclear. The present study assessed the impact of AGE on the Ang-(1-7) axis in NRK-52E renal epithelial cells. MGA exposure for 48 h significantly reduced the intracellular levels of Ang-(1-7) approximately 50%; however, Ang I or Ang II expression was not altered. The reduced cellular content of Ang-(1-7) was associated with increased metabolism of the peptide to the inactive metabolite Ang-(1-4) [MGA: 175±9 vs.
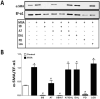
Control: 115±11 fmol/min/mg protein, p<0.05, n=3] but no change in the processing of Ang I to Ang-(1-7). Treatment with Ang-(1-7) reversed MGA-induced cellular hypertrophy and myofibroblast transition evidenced by reduced immunostaining and protein expression of α-smooth muscle actin (α-SMA) [0.4±0.1 vs. 1.0±0.1, respectively, n=3, p<0.05]. Ang-(1-7) abolished AGE-induced activation of the MAP kinase ERK1/2 to a similar extent as the TGF-β receptor kinase inhibitor SB58059; however, Ang-(1-7) did not attenuate the MGA-stimulated release of TGF-β. The AT7-Mas receptor antagonist D-Ala(7)-Ang-(1-7) abolished the inhibitory actions of Ang-(1-7). In contrast, AT1 receptor antagonist losartan did not attenuate the MGA-induced effects. We conclude that Ang-(1-7) may provide an additional therapeutic approach to the conventional RAS blockade regimen to attenuate AGE-dependent renal injury.
Keywords: Advanced glycation endproducts; Angiotensin; EMT; ERK1/2; TGF-B.
Copyright © 2014 Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures








References
-
- Benter IF, Yousif MH, Dhaunsi GS, Kaur J, Chappell MC, Diz DI. Angiotensin-(1-7) prevents activation of NADPH oxidase and renal vascular dysfunction in diabetic hypertensive rats. Am J Nephrol. 2008;28:25–33. - PubMed
-
- Benter IF, Yousif MHM, Anim JT, Cojocel C, Diz DI. Angiotensin-(1-7) prevents development of severe hypertension and end-organ damage in spontaneously hypertensive rats treated with L-NAME. Am J Physiol Heart Circ Physiol. 2006;290:H684–H691. - PubMed
-
- Brenner BM, Cooper ME, de ZD, Keane WF, Mitch WE, Parving HH, Remuzzi G, Snapinn SM, Zhang Z, Shahinfar S. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861–869. - PubMed
-
- Burns WC, Velkoska E, Dean R, Burrell LM, Thomas MC. Angiotensin II mediates epithelial-to-mesenchymal transformation in tubular cells by ANG 1-7/MAS-1-dependent pathways. Am J Physiol Renal Physiol. 2010;299:F585–F593. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

