Modeling putative therapeutic implications of exosome exchange between tumor and immune cells
- PMID: 25246552
- PMCID: PMC4210007
- DOI: 10.1073/pnas.1416745111
Modeling putative therapeutic implications of exosome exchange between tumor and immune cells
Abstract
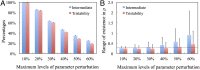
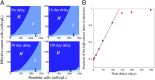
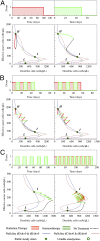
Development of effective strategies to mobilize the immune system as a therapeutic modality in cancer necessitates a better understanding of the contribution of the tumor microenvironment to the complex interplay between cancer cells and the immune response. Recently, effort has been directed at unraveling the functional role of exosomes and their cargo of messengers in this interplay. Exosomes are small vesicles (30-200 nm) that mediate local and long-range communication through the horizontal transfer of information, such as combinations of proteins, mRNAs and microRNAs. Here, we develop a tractable theoretical framework to study the putative role of exosome-mediated cell-cell communication in the cancer-immunity interplay. We reduce the complex interplay into a generic model whose three components are cancer cells, dendritic cells (consisting of precursor, immature, and mature types), and killer cells (consisting of cytotoxic T cells, helper T cells, effector B cells, and natural killer cells). The framework also incorporates the effects of exosome exchange on enhancement/reduction of cell maturation, proliferation, apoptosis, immune recognition, and activation/inhibition. We reveal tristability-possible existence of three cancer states: a low cancer load with intermediate immune level state, an intermediate cancer load with high immune level state, and a high cancer load with low immune-level state, and establish the corresponding effective landscape for the cancer-immunity network. We illustrate how the framework can contribute to the design and assessments of combination therapies.
Keywords: cancer exosomes; cancer landscape; cancer therapy; computational modeling; exosome communication.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271(5256):1734–1736. - PubMed
-
- Mougiakakos D, Choudhury A, Lladser A, Kiessling R, Johansson CC. In: Advances in Cancer Research. Vande Woude GF, Klein G, editors. Academic; New York: 2010. pp. 57–117. - PubMed
-
- Sica A, Schioppa T, Mantovani A, Allavena P. Tumour-associated macrophages are a distinct M2 polarised population promoting tumour progression: Potential targets of anti-cancer therapy. Eur J Cancer. 2006;42(6):717–727. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

