α-Synuclein assembles into higher-order multimers upon membrane binding to promote SNARE complex formation
- PMID: 25246573
- PMCID: PMC4210039
- DOI: 10.1073/pnas.1416598111
α-Synuclein assembles into higher-order multimers upon membrane binding to promote SNARE complex formation
Abstract
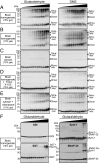
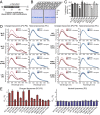
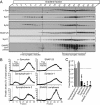
Physiologically, α-synuclein chaperones soluble NSF attachment protein receptor (SNARE) complex assembly and may also perform other functions; pathologically, in contrast, α-synuclein misfolds into neurotoxic aggregates that mediate neurodegeneration and propagate between neurons. In neurons, α-synuclein exists in an equilibrium between cytosolic and membrane-bound states. Cytosolic α-synuclein appears to be natively unfolded, whereas membrane-bound α-synuclein adopts an α-helical conformation. Although the majority of studies showed that cytosolic α-synuclein is monomeric, it is unknown whether membrane-bound α-synuclein is also monomeric, and whether chaperoning of SNARE complex assembly by α-synuclein involves its cytosolic or membrane-bound state. Here, we show using chemical cross-linking and fluorescence resonance energy transfer (FRET) that α-synuclein multimerizes into large homomeric complexes upon membrane binding. The FRET experiments indicated that the multimers of membrane-bound α-synuclein exhibit defined intermolecular contacts, suggesting an ordered array. Moreover, we demonstrate that α-synuclein promotes SNARE complex assembly at the presynaptic plasma membrane in its multimeric membrane-bound state, but not in its monomeric cytosolic state. Our data delineate a folding pathway for α-synuclein that ranges from a monomeric, natively unfolded form in cytosol to a physiologically functional, multimeric form upon membrane binding, and show that only the latter but not the former acts as a SNARE complex chaperone at the presynaptic terminal, and may protect against neurodegeneration.
Keywords: Parkinson's disease; SNARE proteins; membrane fusion; synapse.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- George JM, Jin H, Woods WS, Clayton DF. Characterization of a novel protein regulated during the critical period for song learning in the zebra finch. Neuron. 1995;15(2):361–372. - PubMed
-
- Iwai A, et al. The precursor protein of non-A beta component of Alzheimer’s disease amyloid is a presynaptic protein of the central nervous system. Neuron. 1995;14(2):467–475. - PubMed
-
- Polymeropoulos MH, et al. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science. 1997;276(5321):2045–2047. - PubMed
-
- Krüger R, et al. Ala30Pro mutation in the gene encoding α-synuclein in Parkinson’s disease. Nat Genet. 1998;18(2):106–108. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

