Remotely Activated Mechanotransduction via Magnetic Nanoparticles Promotes Mineralization Synergistically With Bone Morphogenetic Protein 2: Applications for Injectable Cell Therapy
- PMID: 25246698
- PMCID: PMC4214839
- DOI: 10.5966/sctm.2014-0017
Remotely Activated Mechanotransduction via Magnetic Nanoparticles Promotes Mineralization Synergistically With Bone Morphogenetic Protein 2: Applications for Injectable Cell Therapy
Abstract
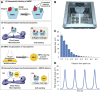
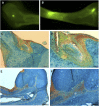
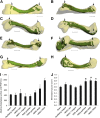
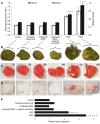
Bone requires dynamic mechanical stimulation to form and maintain functional tissue, yet mechanical stimuli are often lacking in many therapeutic approaches for bone regeneration. Magnetic nanoparticles provide a method for delivering these stimuli by directly targeting cell-surface mechanosensors and transducing forces from an external magnetic field, resulting in remotely controllable mechanotransduction. In this investigation, functionalized magnetic nanoparticles were attached to either the mechanically gated TREK1 K+ channel or the (integrin) RGD-binding domains of human mesenchymal stem cells. These cells were microinjected into an ex vivo chick fetal femur (embryonic day 11) that was cultured organotypically in vitro as a model for endochondral bone formation. An oscillating 25-mT magnetic field delivering a force of 4 pN per nanoparticle directly against the mechanoreceptor induced mechanotransduction in the injected mesenchymal stem cells. It was found that cells that received mechanical stimuli via the nanoparticles mineralized the epiphyseal injection site more extensively than unlabeled control cells. The nanoparticle-tagged cells were also seeded into collagen hydrogels to evaluate osteogenesis in tissue-engineered constructs: in this case, inducing mechanotransduction by targeting TREK1 resulted in a 2.4-fold increase in mineralization and significant increases in matrix density. In both models, the combination of mechanical stimulation and sustained release of bone morphogenetic protein 2 (BMP2) from polymer microspheres showed a significant additive effect on mineralization, increasing the effectiveness of BMP2 delivery and demonstrating that nanoparticle-mediated mechanotransduction can be used synergistically with pharmacological approaches for orthopedic tissue engineering to maximize bone formation.
Keywords: Bone marrow stromal cells; Cellular therapy; Clinical translation; Differentiation; Mesenchymal stem cells; Tissue regeneration; Transduction.
©AlphaMed Press.
Figures

References
-
- Simon JA, Ricci JL, Di Cesare PE. Bioresorbable fracture fixation in orthopedics: A comprehensive review. Part I. Basic science and preclinical studies. Am J Orthop. 1997;26:665–671. - PubMed
-
- Kim IS, Song YM, Cho TH, et al. Synergistic action of static stretching and BMP-2 stimulation in the osteoblast differentiation of C2C12 myoblasts. J Biomech. 2009;42:2721–2727. - PubMed
-
- Griffin XL, Costello I, Costa ML. The role of low intensity pulsed ultrasound therapy in the management of acute fractures: A systematic review. J Trauma. 2008;65:1446–1452. - PubMed
-
- Frias C, Reis J, Capela e Silva F, et al. Polymeric piezoelectric actuator substrate for osteoblast mechanical stimulation. J Biomech. 2010;43:1061–1066. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- bb/g010560/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/G010560/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- MR/K026682/1/MRC_/Medical Research Council/United Kingdom
- bb/g010617/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/G010617/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources

