Benefits of a continuous ambulatory peritoneal dialysis (CAPD) technique with one icodextrin-containing and two biocompatible glucose-containing dialysates for preservation of residual renal function and biocompatibility in incident CAPD patients
- PMID: 25246739
- PMCID: PMC4168174
- DOI: 10.3346/jkms.2014.29.9.1217
Benefits of a continuous ambulatory peritoneal dialysis (CAPD) technique with one icodextrin-containing and two biocompatible glucose-containing dialysates for preservation of residual renal function and biocompatibility in incident CAPD patients
Abstract
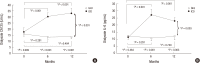
In a prospective randomized controlled study, the efficacy and safety of a continuous ambulatory peritoneal dialysis (CAPD) technique has been evaluated using one icodextrin-containing and two glucose-containing dialysates a day. Eighty incident CAPD patients were randomized to two groups; GLU group continuously using four glucose-containing dialysates (n=39) and ICO group using one icodextrin-containing and two glucose-containing dialysates (n=41). Variables related to residual renal function (RRF), metabolic and fluid control, dialysis adequacy, and dialysate effluent cancer antigen 125 (CA125) and interleukin 6 (IL-6) levels were measured. The GLU group showed a significant decrease in mean renal urea and creatinine clearance (-Δ1.2 ± 2.9 mL/min/1.73 m(2), P=0.027) and urine volume (-Δ363.6 ± 543.0 mL/day, P=0.001) during 12 months, but the ICO group did not (-Δ0.5 ± 2.7 mL/min/1.73 m(2), P=0.266; -Δ108.6 ± 543.3 mL/day, P=0.246). Peritoneal glucose absorption and dialysate calorie load were significantly lower in the ICO group than the GLU group. The dialysate CA125 and IL-6 levels were significantly higher in the ICO group than the GLU group. Dialysis adequacy, β2-microglobulin clearance and blood pressure did not differ between the two groups. The CAPD technique using one icodextrin-containing and two glucose-containing dialysates tends to better preserve RRF and is more biocompatible, with similar dialysis adequacy compared to that using four glucose-containing dialysates in incident CAPD patients. [Clincal Trial Registry, ISRCTN23727549].
Keywords: Biocompatibility; Icodextrin, Randomized Controlled Trial; Peritoneal Dialysis, Continuous Ambulatory; Residual Renal Function.
Conflict of interest statement
The authors have declared that no conflict of interest exists.
Figures


References
-
- Bargman JM, Thorpe KE, Churchill DN CANUSA Peritoneal Dialysis Study Group. Relative contribution of residual renal function and peritoneal clearance to adequacy of dialysis: a reanalysis of the CANUSA Study. J Am Soc Nephrol. 2001;12:2158–2162. - PubMed
-
- Diaz-Buxo JA, Lowrie EG, Lew NL, Zhang SM, Zhu X, Lazarus JM. Associates of mortality among peritoneal dialysis patients with special reference to peritoneal transport rates and solute clearance. Am J Kidney Dis. 1999;33:523–534. - PubMed
-
- Maiorca R, Brunori G, Zubani R, Cancarini GC, Manili L, Camerini C, Movilli E, Pola A, d'Avolio G, Gelatti U. Predictive value of dialysis adequacy and nutritional indices for mortality and morbidity in CAPD and HD patients: a longitudinal study. Nephrol Dial Transplant. 1995;10:2295–2305. - PubMed
-
- Paniagua R, Amato D, Vonesh E, Correa-Rotter R, Ramos A, Moran J, Mujais S Mexican Nephrology Collaborative Study Group. Effects of increased peritoneal clearances on mortality rates in peritoneal dialysis: ADEMEX, a prospective, randomized, controlled trial. J Am Soc Nephrol. 2002;13:1307–1320. - PubMed
-
- Rocco M, Soucie JM, Pastan S, McClellan WM. Peritoneal dialysis adequacy and risk of death. Kidney Int. 2000;58:446–457. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

