Improving effects of chitosan nanofiber scaffolds on osteoblast proliferation and maturation
- PMID: 25246786
- PMCID: PMC4166309
- DOI: 10.2147/IJN.S68012
Improving effects of chitosan nanofiber scaffolds on osteoblast proliferation and maturation
Abstract
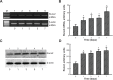
Osteoblast maturation plays a key role in regulating osteogenesis. Electrospun nanofibrous products were reported to possess a high surface area and porosity. In this study, we developed chitosan nanofibers and examined the effects of nanofibrous scaffolds on osteoblast maturation and the possible mechanisms. Macro- and micro observations of the chitosan nanofibers revealed that these nanoproducts had a flat surface and well-distributed fibers with nanoscale diameters. Mouse osteoblasts were able to attach onto the chitosan nanofiber scaffolds, and the scaffolds degraded in a time-dependent manner. Analysis by scanning electron microscopy further showed mouse osteoblasts adhered onto the scaffolds along the nanofibers, and cell-cell communication was also detected. Mouse osteoblasts grew much better on chitosan nanofiber scaffolds than on chitosan films. In addition, human osteoblasts were able to adhere and grow on the chitosan nanofiber scaffolds. Interestingly, culturing human osteoblasts on chitosan nanofiber scaffolds time-dependently increased DNA replication and cell proliferation. In parallel, administration of human osteoblasts onto chitosan nanofibers significantly induced osteopontin, osteocalcin, and alkaline phosphatase (ALP) messenger (m)RNA expression. As to the mechanism, chitosan nanofibers triggered runt-related transcription factor 2 mRNA and protein syntheses. Consequently, results of ALP-, alizarin red-, and von Kossa-staining analyses showed that chitosan nanofibers improved osteoblast mineralization. Taken together, results of this study demonstrate that chitosan nanofibers can stimulate osteoblast proliferation and maturation via runt-related transcription factor 2-mediated regulation of osteoblast-associated osteopontin, osteocalcin, and ALP gene expression.
Keywords: Runx2; chitosan nanofibers; osteoblast maturation; osteoblast-associated gene expression.
Figures






References
-
- Seeman E, Delmas PD. Bone quality – the material and structural basis of bone strength and fragility. New Engl J Med. 2006;354:2250–2261. - PubMed
-
- Ducy P, Schinke T, Karsenty G. The osteoblast: a sophisticated fibroblast under central surveillance. Science. 2000;289:1501–1504. - PubMed
-
- Chen RM, Lin YL, Chou CW. GATA-3 transduces survival signals in osteoblasts through upregulation of bcl-xL gene expression. J Bone Min Res. 2010;25:2193–2204. - PubMed
-
- Aubin JE, Liu F, Malaval L, Gupta AK. Osteoblast and chondroblast differentiation. Bone. 1995;17:77S–83S. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

