Biodegradable drug-eluting nanofiber-enveloped implants for sustained release of high bactericidal concentrations of vancomycin and ceftazidime: in vitro and in vivo studies
- PMID: 25246790
- PMCID: PMC4168873
- DOI: 10.2147/IJN.S66526
Biodegradable drug-eluting nanofiber-enveloped implants for sustained release of high bactericidal concentrations of vancomycin and ceftazidime: in vitro and in vivo studies
Abstract
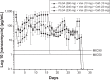
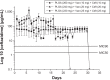
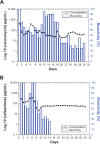
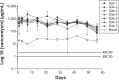
We developed biodegradable drug-eluting nanofiber-enveloped implants that provided sustained release of vancomycin and ceftazidime. To prepare the biodegradable nanofibrous membranes, poly(D,L)-lactide-co-glycolide and the antibiotics were first dissolved in 1,1,1,3,3,3-hexafluoro-2-propanol. They were electrospun into biodegradable drug-eluting membranes, which were then enveloped on the surface of stainless plates. An elution method and a high-performance liquid chromatography assay were employed to characterize the in vivo and in vitro release rates of the antibiotics from the nanofiber-enveloped plates. The results showed that the biodegradable nanofiber-enveloped plates released high concentrations of vancomycin and ceftazidime (well above the minimum inhibitory concentration) for more than 3 and 8 weeks in vitro and in vivo, respectively. A bacterial inhibition test was carried out to determine the relative activity of the released antibiotics. The bioactivity ranged from 25% to 100%. In addition, the serum creatinine level remained within the normal range, suggesting that the high vancomycin concentration did not affect renal function. By adopting the electrospinning technique, we will be able to manufacture biodegradable drug-eluting implants for the long-term drug delivery of different antibiotics.
Keywords: antibiotics; biodegradable nanofiber-enveloped plates; electrospinning; release characteristics.
Figures





References
-
- Struijs PA, Poolman RW, Bhandari M. Infected nonunion of the long bones. J Orthop Trauma. 2007;21(7):507–511. - PubMed
-
- Crowley DJ, Kanakaris NK, Giannoudis PV. Debridement and wound closure of open fractures: the impact of the time factor on infection rates. Injury. 2007;38(8):879–889. - PubMed
-
- Trampuz A, Zimmerli W. Diagnosis and treatment of infections associated with fracture-fixation devices. Injury. 2006;37(Suppl 2):S59–S66. - PubMed
-
- Ueng SW, Wei FC, Shih CH. Management of femoral diaphyseal infected nonunion with antibiotic beads local therapy, external skeletal fixation, and staged bone grafting. J Trauma. 1999;46(1):97–103. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

