Targeting of interleukin-17 in the treatment of psoriasis
- PMID: 25246805
- PMCID: PMC4168861
- DOI: 10.2147/CCID.S67534
Targeting of interleukin-17 in the treatment of psoriasis
Abstract
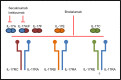
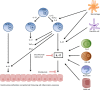
"Psoriasis" is a chronic immune-mediated inflammatory disorder with epidermal hyperplasia. There is some evidence that the cytokine interleukin-17A (often known as IL-17), which is mainly produced by Th17 cells, has a role in the pathogenesis of psoriasis. "IL-17" is a pro-inflammatory cytokine mainly important in the host's defense against extracellular bacteria and fungi. The three new therapies with biologic drugs - brodalumab, secukinumab, and ixekizumab - all target the IL-17 signaling pathway. Secukinumab and ixekizumab neutralize IL-17A, while brodalumab blocks its receptor. Results from clinical trials have shown marked improvements in disease severity in patients with moderate-to-severe plaque psoriasis, using any of these three drugs. The biologic agents were generally well tolerated, but the duration of the trials was relatively short. In this review, we focus on the role of the IL-17 cytokine family in the pathogenesis of psoriasis; the efficacy, safety, and tolerability of brodalumab, secukinumab, and ixekizumab in clinical trials; and possible differences between targeting of the IL-17A receptor and targeting of the IL-17A ligand.
Keywords: IL-17; anti-IL-17 agents; brodalumab; ixekizumab; psoriasis; secukinumab.
Figures
References
-
- Peters BP, Weissman FG, Gill MA. Pathophysiology and treatment of psoriasis. Am J Health Sys Pharm. 2000;57(7):645–659. - PubMed
-
- Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med. 2009;361(5):496–509. - PubMed
-
- Raychaudhuri SP, Farber EM. The prevalence of psoriasis in the world. J Eur Acad Dermatol Venereol. 2001;15(1):16–17. - PubMed
-
- Schäfer T. Epidemiology of psoriasis. Review and the German perspective. Dermatology. 2006;212(4):327–337. - PubMed
-
- Prey S, Paul C, Bronsard V, et al. Assessment of risk of psoriatic arthritis in patients with plaque psoriasis: a systematic review of the literature. J Eur Acad Dermatol Venereol. 2012;24(Suppl 2):31–35. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

