Efficient solar photocatalyst based on cobalt oxide/iron oxide composite nanofibers for the detoxification of organic pollutants
- PMID: 25246877
- PMCID: PMC4169707
- DOI: 10.1186/1556-276X-9-510
Efficient solar photocatalyst based on cobalt oxide/iron oxide composite nanofibers for the detoxification of organic pollutants
Abstract
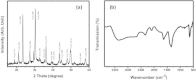
A Co3O4/Fe2O3 composite nanofiber-based solar photocatalyst has been prepared, and its catalytic performance was evaluated by degrading acridine orange (AO) and brilliant cresyl blue (BCB) beneath solar light. The morphological and physiochemical structure of the synthesized solar photocatalyst was characterized by X-ray diffraction (XRD), field emission scanning electron microscopy (FESEM), X-ray photoelectron spectroscopy (XPS), and Fourier transform infrared spectroscopy (FTIR). FESEM indicates that the Co3O4/Fe2O3 composite has fiber-like nanostructures with an average diameter of approximately 20 nm. These nanofibers are made of aggregated nanoparticles having approximately 8.0 nm of average diameter. The optical properties were examined by UV-visible spectrophotometry, and the band gap of the solar photocatalyst was found to be 2.12 eV. The as-grown solar photocatalyst exhibited high catalytic degradation in a short time by applying to degrade AO and BCB. The pH had an effect on the catalytic performance of the as-grown solar photocatalyst, and it was found that the synthesized solar photocatalyst is more efficient at high pH. The kinetics study of both AO and BCB degradation indicates that the as-grown nanocatalyst would be a talented and efficient solar photocatalyst for the removal of hazardous and toxic organic materials.
Keywords: Acridine orange; Brilliant cresyl blue; Co3O4/Fe2O3; Nanofiber; Organic pollutant; Solar photocatalyst.
Figures






References
-
- Khan SB, Lee J-W, Marwani HM, Akhtar K, Asiri AM, Seo J, Khan AAP, Han H. Polybenzimidazole hybrid membranes as a selective adsorbent of mercury. Compos B. 2014;56:392–396.
-
- Jain RK, Kapur M, Labana S, Lal B, Sharma PM, Bhattacharya D, Thakur IS. Microbial diversity: application of microorganisms for the biodegradation of xenobiotics. Curr Sci. 2005;89:101–112.
-
- Lin W-C, Chen C-H, Tang H-Y, Hsiao Y-C, Pan JR, Hu C-C, Huang C. Electrochemical photocatalytic degradation of dye solution with a TiO2-coated stainless steel electrode prepared by electrophoretic deposition. Appl Catal B Environ. 2013;140–141:32–41.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

