Glycoprotein IIb/IIIa and P2Y12 induction by oligochitosan accelerates platelet aggregation
- PMID: 25247182
- PMCID: PMC4163351
- DOI: 10.1155/2014/653149
Glycoprotein IIb/IIIa and P2Y12 induction by oligochitosan accelerates platelet aggregation
Abstract
Platelet membrane receptor glycoprotein IIb/IIIa (gpiibiiia) is a receptor detected on platelets. Adenosine diphosphate (ADP) activates gpiibiiia and P2Y12, causing platelet aggregation and thrombus stabilization during blood loss. Chitosan biomaterials were found to promote surface induced hemostasis and were capable of activating blood coagulation cascades by enhancing platelet aggregation. Our current findings show that the activation of the gpiibiiia complex and the major ADP receptor P2Y12 is required for platelet aggregation to reach hemostasis following the adherence of various concentrations of chitosan biomaterials [7% N,O-carboxymethylchitosan (NO-CMC) with 0.45 mL collagen, 8% NO-CMC, oligochitosan (O-C), and oligochitosan 53 (O-C 53)]. We studied gpiibiiia and P2Y12 through flow cytometric analysis and western blotting techniques. The highest expression of gpiibiiia was observed with Lyostypt (74.3 ± 7.82%), followed by O-C (65.5 ± 7.17%). Lyostypt and O-C resulted in gpiibiiia expression increases of 29.2% and 13.9%, respectively, compared with blood alone. Western blot analysis revealed that only O-C 53 upregulated the expression of P2Y12 (1.12 ± 0.03-fold) compared with blood alone. Our findings suggest that the regulation of gpiibiiia and P2Y12 levels could be clinically useful to activate platelets to reach hemostasis. Further, we show that the novel oligochitosan is able to induce the increased expression of gpiibiiia and P2Y12, thus accelerating platelet aggregation in vitro.
Figures

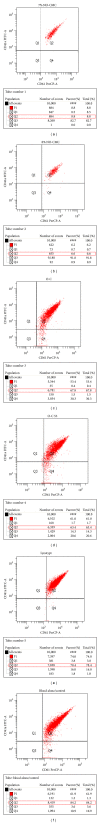

References
-
- Nakamura T, Kambayashi J, Okuma M, Tandon NN. Activation of the GP IIb-IIIa complex induced by platelet adhesion to collagen is mediated by both α2β1 integrin and GP VI. The Journal of Biological Chemistry. 1999;274(17):11897–11903. - PubMed
-
- Vickers JD. Binding of polymerizing fibrin to integrin α(IIb)β3 on chymotrypsin-treated rabbit platelets decreases phosphatidylinositol 4,5-bisphosphate and increases cytoskeletal actin. Platelets. 1999;10(4):228–237. - PubMed
-
- Calvete JJ. On the structure and function of platelet integrin α(IIb)β3, the fibrinogen receptor. Proceedings of the Society for Experimental Biology and Medicine. 1995;208(4):346–360. - PubMed
-
- Shattil SJ. Signaling through platelet integrin αIIb β 3: Inside-out, outside-in, and sideways. Thrombosis and Haemostasis. 1999;82(2):318–325. - PubMed
-
- Gachet C. ADP receptors of platelets and their inhibition. Thrombosis and Haemostasis. 2001;86(1):222–232. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

