High systemic levels of the cytokine-inducing HMGB1 isoform secreted in severe macrophage activation syndrome
- PMID: 25247290
- PMCID: PMC4365070
- DOI: 10.2119/molmed.2014.00183
High systemic levels of the cytokine-inducing HMGB1 isoform secreted in severe macrophage activation syndrome
Retraction in
-
Retraction Note to: High systemic levels of the cytokine-inducing HMGB1 isoform secreted in severe macrophage activation syndrome.Mol Med. 2020 Dec 30;26(1):131. doi: 10.1186/s10020-020-00263-2. Mol Med. 2020. PMID: 33380314 Free PMC article. No abstract available.
Expression of concern in
-
Expression of concern to: High systemic levels of the cytokine-inducing HMGB1 isoform secreted in severe macrophage activation syndrome.Mol Med. 2020 Feb 3;26(1):17. doi: 10.1186/s10020-020-0142-x. Mol Med. 2020. PMID: 32013863 Free PMC article. No abstract available.
Abstract
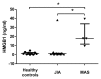
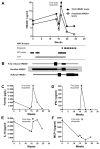
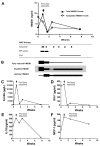
Macrophage activation syndrome (MAS) is a potentially fatal complication of systemic inflammation. High mobility group box 1 (HMGB1) is a nuclear protein extensively leaked extracellularly during necrotic cell death or actively secreted by natural killer (NK) cells, macrophages and additional cells during infection or sterile injury. Extracellular HMGB1 orchestrates key events in inflammation as a prototypic alarmin. The redox states of its three cysteines render the molecule mutually exclusive functions: fully reduced "all-thiol HMGB1" exerts chemotactic activity; "disulfide HMGB1" has cytokine-inducing, toll-like receptor 4 (TLR4)-mediated effects—while terminally oxidized "sulfonyl HMGB1" lacks inflammatory activity. This study examines the kinetic pattern of systemic HMGB1 isoform expression during therapy in four children with severe MAS. Three of the four patients with underlying systemic rheumatic diseases were treated with biologics and two suffered from triggering herpes virus infections at the onset of MAS. All patients required intensive care unit therapy due to life-threatening illness. Tandem mass-spectrometric analysis revealed dramatically increased systemic levels of the cytokine-inducing HMGB1 isoform during early MAS. Disease control coincided with supplementary etoposide therapy initiated to boost apoptotic cell death, when systemic HMGB1 levels drastically declined and the molecule emerged mainly in its oxidized, noninflammatory isoform. Systemic interferon (IFN)-γ and ferritin peaked concomitantly with HMGB1, whereas interleukin (IL)-18 and monocyte chemotactic protein (MCP)-1 levels developed differently. In conclusion, this work provides new insights in HMGB1 biology, suggesting that the molecule is not merely a biomarker of inflammation, but most likely also contributes to the pathogenesis of MAS. These observations encourage further studies of disulfide HMGB1 antagonists to improve outcome of MAS.
Figures


References
-
- Ravelli A, Grom AA, Behrens EM, Cron RQ. Macrophage activation syndrome as part of systemic juvenile idiopathic arthritis: diagnosis, genetics, pathophysiology and treatment. Genes Immun. 2012;13:289–98. - PubMed
-
- Parodi A, et al. Macrophage activation syndrome in juvenile systemic lupus erythematosus: a multinational multicenter study of thirty-eight patients. Arthritis Rheum. 2009;60:3388–99. - PubMed
-
- Simonini G, et al. Macrophage activation syndrome/hemophagocytic lymphohistiocytosis and Kawasaki disease. Ped Blood Canc. 2010;55:592. - PubMed
-
- Athreya BH. Is macrophage activation syndrome a new entity? Clin Exp Rheumatol. 2002;20:121–3. - PubMed
-
- Ramanan AV, Schneider R. Macrophage activation syndrome—what’s in a name! J Rheumatol. 2003;30:2513–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

