Dose escalation to rash for erlotinib plus gemcitabine for metastatic pancreatic cancer: the phase II RACHEL study
- PMID: 25247318
- PMCID: PMC4260026
- DOI: 10.1038/bjc.2014.494
Dose escalation to rash for erlotinib plus gemcitabine for metastatic pancreatic cancer: the phase II RACHEL study
Abstract
Background: This phase II, open-label, randomised study evaluated whether patients with metastatic pancreatic cancer receiving erlotinib/gemcitabine derived survival benefits from increasing the erlotinib dose.
Methods: After a 4-week run-in period (gemcitabine 1000 mg m(-2) once weekly plus erlotinib 100 mg per day), patients with metastatic pancreatic cancer who developed grade 0/1 rash were randomised to receive gemcitabine plus erlotinib dose escalation (150 mg, increasing by 50 mg every 2 weeks (maximum 250 mg); n=71) or gemcitabine plus standard-dose erlotinib (100 mg per day; n=75). The primary end point was to determine whether overall survival (OS) was improved by increasing the erlotinib dose. Secondary end points included progression-free survival (PFS), incidence of grade ⩾2 rash, and safety.
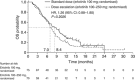
Results: Erlotinib dose escalation induced grade ⩾2 rash in 29 out of 71 (41.4%) patients compared with 7 out of 75 (9.3%) patients on standard dose. Efficacy was not significantly different in the dose-escalation arm compared with the standard-dose arm (OS: median 7.0 vs 8.4 months, respectively, hazard ratio (HR), 1.26, 95% confidence interval (CI): 0.88-1.80; P=0.2026; PFS: median 3.5 vs 4.5 months, respectively, HR, 1.09, 95% CI: 0.77-1.54; P=0.6298). Incidence of adverse events was comparable between randomised arms.
Conclusion: The erlotinib dose-escalation strategy induced rash in some patients; there was no evidence that the higher dose translated into increased benefit.
Figures


References
-
- Alejandro LM, Adel NG, O'Reilly EM, Riedel E, Lacouture ME. Association between skin toxicities and treatment outcomes in patients with pancreatic cancer (PC) receiving erlotinib (E): Memorial Sloan-Kettering Cancer Center (MSKCC) experience. J Clin Oncol. 2012;30:Abstract 294.
-
- Amador MR, Oppenheimer D, Perea S, Maitra A, Cusatis G, Iacobuzio-Donahue C, Baker SD, Ashfaq R, Takimoto C, Forastiere A, Hidalgo M. An epidermal growth factor receptor intro 1 polymorphism mediates response to epidermal growth factor receptor inhibitors. Cancer Res. 2004;64:9139–9143. - PubMed
-
- Aranda E, Manzano JL, Rivera F, Galán M, Valladares-Ayerbes M, Pericay C, Safont MJ, Mendez MJ, Irigoyen A, Arrivi A, Sastre J, Díaz-Rubio E. Phase II open-label study of erlotinib in combination with gemcitabine in unresectable and/or metastatic adenocarcinoma of the pancreas: relationship between skin rash and survival (Pantar study) Ann Oncol. 2012;23:1919–1925. - PubMed
-
- Buges C, Marti AM, Rosell R, Vergnenegre A, De Marinis F, Massuti B, De Castro J, Gervais R, Costa EC, Moran T, Santarpia M, Felip E, Majem M, Porta R, Palmero R, Drozdowskyj A, Heidecke C, Gasco A, Taron M, Paz-Ares LG. Skin toxicity associated with outcome to erlotinib in non-small cell lung cancer (NSCLC) patients (p) with EGFR mutations in the EURTAC study. J Clin Oncol. 2012;30:Abstract 7542.
-
- Burris H, 3rd, Rocha-Lima C. New therapeutic directions for advanced pancreatic cancer: targeting the epidermal growth factor and vascular endothelial growth factor pathways. Oncologist. 2008;13:289–298. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

