Efficacy of the irreversible ErbB family blocker afatinib in epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI)-pretreated non-small-cell lung cancer patients with brain metastases or leptomeningeal disease
- PMID: 25247337
- PMCID: PMC4276567
- DOI: 10.1097/JTO.0000000000000380
Efficacy of the irreversible ErbB family blocker afatinib in epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI)-pretreated non-small-cell lung cancer patients with brain metastases or leptomeningeal disease
Abstract
Introduction: Afatinib is an effective first-line treatment in patients with epidermal growth factor receptor (EGFR)-mutated non-small-cell lung cancer (NSCLC) and has shown activity in patients progressing on EGFR-tyrosine kinase inhibitors (TKIs). First-line afatinib is also effective in patients with central nervous system (CNS) metastasis. Here we report on outcomes of pretreated NSCLC patients with CNS metastasis who received afatinib within a compassionate use program.
Methods: Patients with NSCLC progressing after at least one line of chemotherapy and one line of EGFR-TKI treatment received afatinib. Medical history, patient demographics, EGFR mutational status, and adverse events including tumor progression were documented.
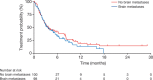
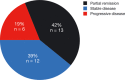
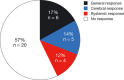
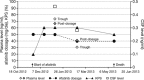
Results: From 2010 to 2013, 573 patients were enrolled and 541 treated with afatinib. One hundred patients (66% female; median age, 60 years) had brain metastases and/or leptomeningeal disease with 74% having documented EGFR mutation. Median time to treatment failure for patients with CNS metastasis was 3.6 months, and did not differ from a matched group of 100 patients without CNS metastasis. Thirty-five percent (11 of 31) of evaluable patients had a cerebral response, five (16%) responded exclusively in brain. Response duration (range) was 120 (21-395) days. Sixty-six percent (21 of 32) of patients had cerebral disease control on afatinib. Data from one patient with an impressive response showed an afatinib concentration in the cerebrospinal fluid of nearly 1 nMol.
Conclusion: Afatinib appears to penetrate into the CNS with concentrations high enough to have clinical effect on CNS metastases. Afatinib may therefore be an effective treatment for heavily pretreated patients with EGFR-mutated or EGFR-TKI-sensitive NSCLC and CNS metastasis.
Conflict of interest statement
Drs. Hoffknecht and Tufman contributed equally to this work.
Figures
Comment in
-
Afatinib for Erlotinib Refractory Brain Metastases in a Patient with EGFR-Mutant Non-Small-Cell Lung Cancer: Can High-Affinity TKI Substitute for High-Dose TKI?J Thorac Oncol. 2015 Jul;10(7):e65-6. doi: 10.1097/JTO.0000000000000479. J Thorac Oncol. 2015. PMID: 26134239 No abstract available.
References
-
- Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353:123–132. - PubMed
-
- Sharma SV, Bell DW, Settleman J, et al. Epidermal growth factor receptor mutations in lung cancer. Nat Rev Cancer. 2007;7:169–181. - PubMed
-
- Doroshow JH. Targeting EGFR in non-small-cell lung cancer. N Engl J Med. 2005;353:200–202. - PubMed
-
- Johnson BE, Jänne PA. Epidermal growth factor receptor mutations in patients with non-small cell lung cancer. Cancer Res. 2005;65:7525–7529. - PubMed
-
- Pao W. Defining clinically relevant molecular subsets of lung cancer. Cancer Chemother Pharmacol. 2006;58(Suppl 1):s11–s15. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

