Repair and tissue engineering techniques for articular cartilage
- PMID: 25247412
- PMCID: PMC4629810
- DOI: 10.1038/nrrheum.2014.157
Repair and tissue engineering techniques for articular cartilage
Abstract
Chondral and osteochondral lesions due to injury or other pathology commonly result in the development of osteoarthritis, eventually leading to progressive total joint destruction. Although current progress suggests that biologic agents can delay the advancement of deterioration, such drugs are incapable of promoting tissue restoration. The limited ability of articular cartilage to regenerate renders joint arthroplasty an unavoidable surgical intervention. This Review describes current, widely used clinical repair techniques for resurfacing articular cartilage defects; short-term and long-term clinical outcomes of these techniques are discussed. Also reviewed is a developmental pipeline of acellular and cellular regenerative products and techniques that could revolutionize joint care over the next decade by promoting the development of functional articular cartilage. Acellular products typically consist of collagen or hyaluronic-acid-based materials, whereas cellular techniques use either primary cells or stem cells, with or without scaffolds. Central to these efforts is the prominent role that tissue engineering has in translating biological technology into clinical products; therefore, concomitant regulatory processes are also discussed.
Conflict of interest statement
Competing interests
A.H.G. declares that he consults for SANOFI S.A. The other authors declare no competing interests.
Figures

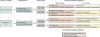

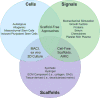
References
-
- Lories RJ, Luyten FP. The bone–cartilage unit in osteoarthritis. Nat Rev Rheumatol. 2011;7:43–49. - PubMed
-
- Moran CJ, et al. Restoration of articular cartilage. J Bone Joint Surg Am. 2014;96:336–344. - PubMed
-
- Bae DK, Yoon KH, Song SJ. Cartilage healing after microfracture in osteoarthritic knees. Arthroscopy. 2006;22:367–374. - PubMed
-
- Kreuz PC, et al. Results after microfracture of full-thickness chondral defects in different compartments in the knee. Osteoarthritis Cartilage. 2006;14:1119–1125. - PubMed
-
- Mithoefer K, McAdams T, Williams RJ, Kreuz PC, Mandelbaum BR. Clinical efficacy of the microfracture technique for articular cartilage repair in the knee: an evidence-based systematic analysis. Am J Sports Med. 2009;37:2053–2063. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

