Both carboplatin and bevacizumab improve pathological complete remission rate in neoadjuvant treatment of triple negative breast cancer: a meta-analysis
- PMID: 25247558
- PMCID: PMC4172579
- DOI: 10.1371/journal.pone.0108405
Both carboplatin and bevacizumab improve pathological complete remission rate in neoadjuvant treatment of triple negative breast cancer: a meta-analysis
Abstract
Triple negative breast cancer (TNBC) is associated with high pathological complete remission (pCR) rate in neoadjuvant treatment (NAT). TNBC patients who achieve pCR have superior outcome than those without pCR. A meta-analysis was done to evaluate whether integrating novel approaches into NAT can improve the pCR rate in TNBC. Medical subject heading terms (Breast Neoplasm) and key words (triple negative OR estrogen receptor (ER) negative OR HER2 negative) AND (primary systemic OR neoadjuvant OR preoperative) were used to select eligible studies. Experimental arm in each study was considered as the testing regimen, and control arm was defined as the standard regimen in this meta-analysis. A total of 11 studies with 14 paired regimens were included in the final analysis. Aggregate pCR rate was 37.3% and 44.6% in the standard and testing group, respectively. Novel approaches in the testing regimen significantly improved the pCR rate in NAT of TNBC patients compared with the standard regimen, with an odds ratio (OR) of 1.34 (95% confidence interval (CI) 1.11-1.62, P = 0.002). Considering specific regimens, we demonstrated the pCR rate to be much higher in the carboplatin-containing (OR = 1.80, 95% CI 1.39-2.32, P<0.001) or bevacizumab-containing regimens (OR = 1.36, 95% CI 1.11-1.66, P = 0.003) than in the control regimens. The addition of carboplatin in NAT had a pCR rate as high as 51.2% in TNBC patients, with an absolute pCR difference of 13.8% as compared with control regimens. No significant heterogeneity was identified among studies evaluating the addition of carboplatin or bevacizumab efficacy in NAT. This meta-analysis indicates that these novel NAT regimens have achieved a significant pCR improvement in TNBC patients, especially among patients treated with carboplatin-containing or bevacizumab-containing regimen. This can help us design appropriate trials in the adjuvant setting and guide clinical practice.
Conflict of interest statement
Figures
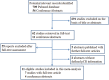

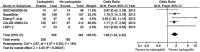

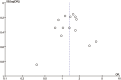
References
-
- Chia S, Swain SM, Byrd DR, Mankoff DA (2008) Locally advanced and inflammatory breast cancer. J Clin Oncol 26: 786–790. - PubMed
-
- Kaufmann M, Hortobagyi GN, Goldhirsch A, Scholl S, Makris A, et al. (2006) Recommendations from an international expert panel on the use of neoadjuvant (primary) systemic treatment of operable breast cancer: an update. J Clin Oncol 24: 1940–1949. - PubMed
-
- Kaufmann M, von Minckwitz G, Bear HD, Buzdar A, McGale P, et al. (2007) Recommendations from an international expert panel on the use of neoadjuvant (primary) systemic treatment of operable breast cancer: new perspectives 2006. Ann Oncol 18: 1927–1934. - PubMed
-
- Rastogi P, Anderson SJ, Bear HD, Geyer CE, Kahlenberg MS, et al. (2008) Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol 26: 778–785. - PubMed
-
- Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, et al. (2000) Molecular portraits of human breast tumours. Nature 406: 747–752. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

