CT and MR imaging diagnosis and staging of hepatocellular carcinoma: part II. Extracellular agents, hepatobiliary agents, and ancillary imaging features
- PMID: 25247563
- PMCID: PMC4263770
- DOI: 10.1148/radiol.14132362
CT and MR imaging diagnosis and staging of hepatocellular carcinoma: part II. Extracellular agents, hepatobiliary agents, and ancillary imaging features
Abstract
Computed tomography (CT) and magnetic resonance (MR) imaging play critical roles in the diagnosis and staging of hepatocellular carcinoma (HCC). The second article of this two-part review discusses basic concepts of diagnosis and staging, reviews the diagnostic performance of CT and MR imaging with extracellular contrast agents and of MR imaging with hepatobiliary contrast agents, and examines in depth the major and ancillary imaging features used in the diagnosis and characterization of HCC.
© RSNA, 2014.
Figures
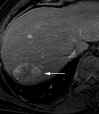






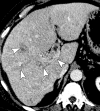

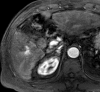
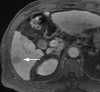
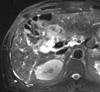

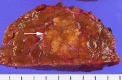
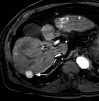
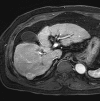


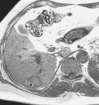
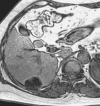
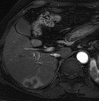

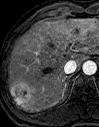

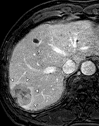
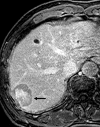
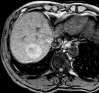






References
-
- Lencioni R, Piscaglia F, Bolondi L. Contrast-enhanced ultrasound in the diagnosis of hepatocellular carcinoma. J Hepatol 2008;48(5):848–857. - PubMed
-
- Kim SR, Ando K, Mita K, et al. . Superiority of CT arterioportal angiography to contrast-enhanced CT and MRI in the diagnosis of hepatocellular carcinoma in nodules smaller than 2 cm. Oncology 2007;72(Suppl 1):58–66. - PubMed
-
- Yoo HJ, Lee JM, Lee JY, et al. . Additional value of SPIO-enhanced MR imaging for the noninvasive imaging diagnosis of hepatocellular carcinoma in cirrhotic liver. Invest Radiol 2009;44(12):800–807. - PubMed
-
- Kim BK, Kang WJ, Kim JK, et al. . 18F-fluorodeoxyglucose uptake on positron emission tomography as a prognostic predictor in locally advanced hepatocellular carcinoma. Cancer 2011;117(20):4779–4787. - PubMed
-
- Bruix J, Sherman M; Practice Guidelines Committee, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma. Hepatology 2005;42(5):1208–1236. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

