Macrophage-expressed perforins mpeg1 and mpeg1.2 have an anti-bacterial function in zebrafish
- PMID: 25247677
- PMCID: PMC6738794
- DOI: 10.1159/000366103
Macrophage-expressed perforins mpeg1 and mpeg1.2 have an anti-bacterial function in zebrafish
Abstract
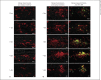
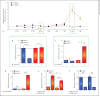
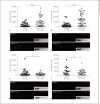
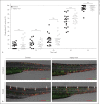
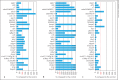
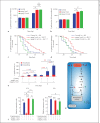
Macrophage-expressed gene 1 (MPEG1) encodes an evolutionarily conserved protein with a predicted membrane attack complex/perforin domain associated with host defence against invading pathogens. In vertebrates, MPEG1/perforin-2 is an integral membrane protein of macrophages, suspected to be involved in the killing of intracellular bacteria by pore-forming activity. Zebrafish have 3 copies of MPEG1; 2 are expressed in macrophages, whereas the third could be a pseudogene. The mpeg1 and mpeg1.2 genes show differential regulation during infection of zebrafish embryos with the bacterial pathogens Mycobacterium marinum and Salmonella typhimurium. While mpeg1 is downregulated during infection with both pathogens, mpeg1.2 is infection inducible. Upregulation of mpeg1.2 is partially dependent on the presence of functional Mpeg1 and requires the Toll-like receptor adaptor molecule MyD88 and the transcription factor NFκB. Knockdown of mpeg1 alters the immune response to M. marinum infection and results in an increased bacterial burden. In Salmonella typhimurium infection, both mpeg1 and mpeg1.2 knockdown increase the bacterial burdens, but mpeg1 morphants show increased survival times. The combined results of these two in vivo infection models support the anti-bacterial function of the MPEG1/perforin-2 family and indicate that the intricate cross-regulation of the two mpeg1 copies aids the zebrafish host in combatting infection of various pathogens.
© 2014 S. Karger AG, Basel.
Figures


References
-
- Kondos SC, Hatfaludi T, Voskoboinik I, Trapani JA, Law RHP, Whisstock JC, Dunstone MA. The structure and function of mammalian membrane-attack complex/perforin-like proteins. Tissue Antigens. 2010;76:341–351. - PubMed
-
- Voskoboinik I, Dunstone MA, Baran K, Whisstock JC, Trapani JA. Perforin: structure, function, and role in human immunopathology. Immunol Rev. 2010;235:35–54. - PubMed
-
- Spilsbury K, O'Mara MA, Wu WM, Rowe PB, Symonds G, Takayama Y. Isolation of a novel macrophage-specific gene by differential cDNA analysis. Blood. 1995;85:1620–1629. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

