APP is cleaved by Bace1 in pre-synaptic vesicles and establishes a pre-synaptic interactome, via its intracellular domain, with molecular complexes that regulate pre-synaptic vesicles functions
- PMID: 25247712
- PMCID: PMC4172690
- DOI: 10.1371/journal.pone.0108576
APP is cleaved by Bace1 in pre-synaptic vesicles and establishes a pre-synaptic interactome, via its intracellular domain, with molecular complexes that regulate pre-synaptic vesicles functions
Abstract
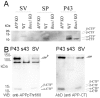
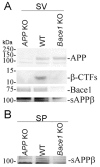
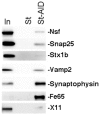
Amyloid Precursor Protein (APP) is a type I membrane protein that undergoes extensive processing by secretases, including BACE1. Although mutations in APP and genes that regulate processing of APP, such as PSENs and BRI2/ITM2B, cause dementias, the normal function of APP in synaptic transmission, synaptic plasticity and memory formation is poorly understood. To grasp the biochemical mechanisms underlying the function of APP in the central nervous system, it is important to first define the sub-cellular localization of APP in synapses and the synaptic interactome of APP. Using biochemical and electron microscopy approaches, we have found that APP is localized in pre-synaptic vesicles, where it is processed by Bace1. By means of a proteomic approach, we have characterized the synaptic interactome of the APP intracellular domain. We focused on this region of APP because in vivo data underline the central functional and pathological role of the intracellular domain of APP. Consistent with the expression of APP in pre-synaptic vesicles, the synaptic APP intracellular domain interactome is predominantly constituted by pre-synaptic, rather than post-synaptic, proteins. This pre-synaptic interactome of the APP intracellular domain includes proteins expressed on pre-synaptic vesicles such as the vesicular SNARE Vamp2/Vamp1 and the Ca2+ sensors Synaptotagmin-1/Synaptotagmin-2, and non-vesicular pre-synaptic proteins that regulate exocytosis, endocytosis and recycling of pre-synaptic vesicles, such as target-membrane-SNAREs (Syntaxin-1b, Syntaxin-1a, Snap25 and Snap47), Munc-18, Nsf, α/β/γ-Snaps and complexin. These data are consistent with a functional role for APP, via its carboxyl-terminal domain, in exocytosis, endocytosis and/or recycling of pre-synaptic vesicles.
Conflict of interest statement
Figures





References
-
- Scheinfeld MH, Ghersi E, Laky K, Fowlkes BJ, D'Adamio L (2002) Processing of beta-amyloid precursor-like protein-1 and -2 by gamma-secretase regulates transcription. J Biol Chem 277: 44195–44201. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

