Characterization of commercially available vaginal lubricants: a safety perspective
- PMID: 25247884
- PMCID: PMC4190534
- DOI: 10.3390/pharmaceutics6030530
Characterization of commercially available vaginal lubricants: a safety perspective
Abstract
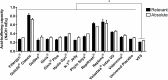
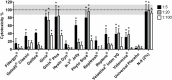
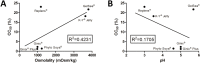
Vaginal lubricants are widely used by women to help solve intercourse difficulties or as enhancers, but recent reports raise questions about their safety. Twelve commercially available gel products were tested for pH value, pH buffering capacity, osmolality and cytotoxicity relevant to vaginal delivery. Obtained data were analyzed in light of the recent Advisory Note by the World Health Organization (WHO) for personal lubricants to be concomitantly used with condoms. Results showed that most products do not comply with pH and osmolality recommended standards, thus posing a potential hazard. Four products presented values of osmolality around three-times higher than the maximum acceptable limit of 1200 mOsm/kg. In vitro cell testing further identified substantial cytotoxicity even at 1:100 dilutions for three products, contrasting with no significant effect of up to at least a 1:5 dilution of a Universal Placebo gel. However, no direct correlation between these last results and pH or osmolality was found, thus suggesting that the individual toxicity of specific formulation components plays an important role in the outcome of a particular product. Although further assessment is required, these results highlight potential safety issues related to the formulation of commercially available vaginal lubricants.
Figures

References
-
- das Neves J., Amaral M.H., Bahia M.F. Vaginal drug delivery. In: Gad S.C., editor. Pharmaceutical Manufacturing Handbook: Production and Processes. Wiley; Hoboken, NJ, USA: 2008. pp. 809–878.
-
- das Neves J., Palmeira-de-Oliveira R., Palmeira-de-Oliveira A., Rodrigues F., Sarmento B. Vaginal mucosa and drug delivery. In: Khutoryanskiy V.V., editor. Mucoadhesive Materials and Drug Delivery Systems. Wiley; Chichester, UK: 2014. pp. 99–131.
-
- Pavelić Ž., Škalko-Basnet N., Jalšenjak I. Characterisation and in vitro evaluation of bioadhesive liposome gels for local therapy of vaginitis. Int. J. Pharm. 2005;301:140–148. - PubMed
-
- das Neves J., Bahia M.F. Gels as vaginal drug delivery systems. Int. J. Pharm. 2006;318:1–14. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

