Crystal structure of patatin-17 in complex with aged and non-aged organophosphorus compounds
- PMID: 25248161
- PMCID: PMC4172759
- DOI: 10.1371/journal.pone.0108245
Crystal structure of patatin-17 in complex with aged and non-aged organophosphorus compounds
Abstract
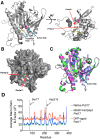
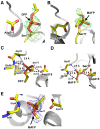
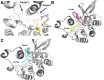
Patatin is a non-specific plant lipase and the eponymous member of a broad class of serine hydrolases termed the patatin-like phospholipase domain containing proteins (PNPLAs). Certain PNPLA family members can be inhibited by organophosphorus (OP) compounds. Currently, no structural data are available on the modes of interaction between the PNPLAs and OP compounds or their native substrates. To this end, we present the crystal structure of patatin-17 (pat17) in its native state as well as following inhibition with methyl arachidonyl fluorophosphonate (MAFP) and inhibition/aging with diisopropylphosphorofluoridate (DFP). The native pat17 structure revealed the existence of two portals (portal1 and portal2) that lead to its active-site chamber. The DFP-inhibited enzyme underwent the aging process with the negatively charged phosphoryl oxygen, resulting from the loss of an isopropyl group, being within hydrogen-binding distance to the oxyanion hole. The MAFP-inhibited pat17 structure showed that MAFP did not age following its interaction with the nucleophilic serine residue (Ser77) of pat17 since its O-methyl group was intact. The MAFP moiety is oriented with its phosphoryl oxygen in close proximity to the oxyanion hole of pat17 and its O-methyl group located farther away from the oxyanion hole of pat17 relative to the DFP-bound state. The orientation of the alkoxy oxygens within the two OP compounds suggests a role for the oxyanion hole in stabilizing the emerging negative charge on the oxygen during the aging reaction. The arachidonic acid side chain of MAFP could be contained within portals 1 or 2. Comparisons of pat17 in the native, inhibited, and aged states showed no significant global conformational changes with respect to their Cα backbones, consistent with observations from other α/β hydrolases such as group VIIA phospholipase A2.
Conflict of interest statement
Figures
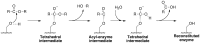

References
-
- Hirayama O, Matsuda H, Takeda H, Maenaka K, Takatsuka H (1975) Purification and properties of a lipid acyl-hydrolase from potato tubers. Biochim Biophys Acta 384: 127–137. - PubMed
-
- Ganal MW, Bonierbale MW, Roeder MS, Park WD, Tanksley SD (1991) Genetic and physical mapping of the patatin genes in potato and tomato. Mol Gen Genet 225: 501–509. - PubMed
-
- Pots AM, Gruppen H, Hessing M, van Boekel MA, Voragen AG (1999) Isolation and characterization of patatin isoforms. J Agric Food Chem 47: 4587–4592. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

