Diagnostic tools for alzheimer's disease dementia and other dementias: an overview of diagnostic test accuracy (DTA) systematic reviews
- PMID: 25248284
- PMCID: PMC4189736
- DOI: 10.1186/s12883-014-0183-2
Diagnostic tools for alzheimer's disease dementia and other dementias: an overview of diagnostic test accuracy (DTA) systematic reviews
Abstract
Background: Dementia includes a group of neurodegenerative disorders characterized by progressive loss of cognitive function and a decrease in the ability to perform activities of daily living. Systematic reviews of diagnostic test accuracy (DTA) focus on how well the index test detects patients with the disease in terms of figures such as sensitivity and specificity. Although DTA reviews about dementia are essential, at present there is no information about their quantity and quality.
Methods: We searched for DTA reviews in MEDLINE (1966-2013), EMBASE (1980-2013), The Cochrane Library (from its inception until December 2013) and the Database of Abstracts of Reviews of Effects (DARE). Two reviewers independently assessed the methodological quality of the reviews using the AMSTAR measurement tool, and the quality of the reporting using the PRISMA checklist. We describe the main characteristics of these reviews, including basic characteristics, type of dementia, and diagnostic test evaluated, and we summarize the AMSTAR and PRISMA scores.
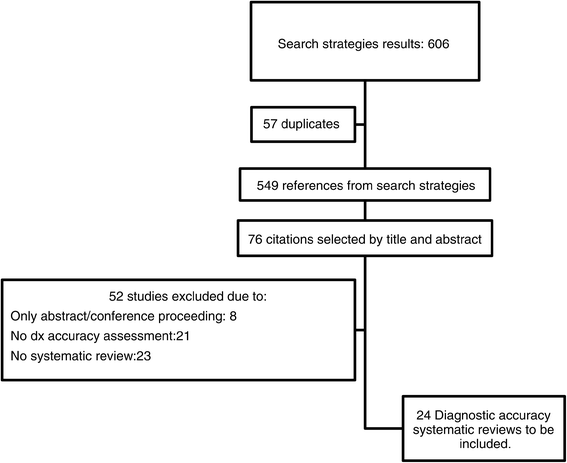
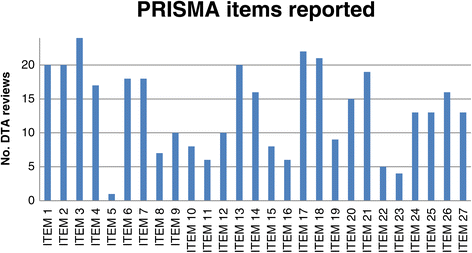
Results: We selected 24 DTA systematic reviews. Only 10 reviews (41.6%), assessed the bias of included studies and few (33%) used this information to report the review results or to develop their conclusions Only one review (4%) reported all methodological items suggested by the PRISMA tool. Assessing methodology quality by means of the AMSTAR tool, we found that six DTA reviews (25%) pooled primary data with the aid of methods that are used for intervention reviews, such as Mantel-Haenszel and separate random-effects models (25%), while five reviews (20.8%) assessed publication bias by means of funnel plots and/or Egger's Test.
Conclusions: Our assessment of these DTA reviews reveals that their quality, both in terms of methodology and reporting, is far from optimal. Assessing the quality of diagnostic evidence is fundamental to determining the validity of the operating characteristics of the index test and its usefulness in specific settings. The development of high quality DTA systematic reviews about dementia continues to be a challenge.
Figures

References
-
- Davis Daniel HJ, Creavin Sam T, Noel-Storr A, Quinn Terry J, Smailagic N, Hyde C, Brayne C, McShane R, Cullum S. Neuropsychological tests for the diagnosis of Alzheimer’s disease dementia and other dementias: a generic protocol for cross-sectional and delayed-verification studies. Cochrane Database Syst Rev. 2013;3:CD010460. - PMC - PubMed
-
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders (DSM-IV) Washington DC: American Psychiatric Association; 1994.
-
- Jack CR, Jr, Albert MS, Knopman DS, McKhann GM, Sperling RA, Carrillo MC, Thies B, Phelps CH. Introduction to the recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):257–262. doi: 10.1016/j.jalz.2011.03.004. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

