Hepatitis B virus vaccination booster does not provide additional protection in adolescents: a cross-sectional school-based study
- PMID: 25248369
- PMCID: PMC4246462
- DOI: 10.1186/1471-2458-14-991
Hepatitis B virus vaccination booster does not provide additional protection in adolescents: a cross-sectional school-based study
Abstract
Background: Current consensus does not support the use of a universal booster of hepatitis B virus (HBV) vaccine because there is an anamnestic response in almost all children 15 years after universal infant HBV vaccination. We aimed to provide a booster strategy among adolescents as a result of their changes in lifestyle and sexual activity.
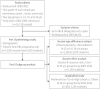
Methods: This study comprised a series of cross-sectional serological surveys of HBV markers in four age groups between 2004 and 2012. The seropositivity rates of hepatitis B surface antigen (HBsAg) and its reciprocal antibody (anti-HBs) for each age group were collected. There were two parts to this study; age-specific HBV seroepidemiology and subgroup analysis, including effects of different vaccine types, booster response for immunogenicity at 15 years of age, and longitudinal follow-up to identify possible additional protection by HBV booster.
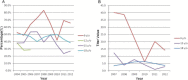
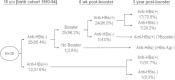
Results: Within the study period, data on serum anti-HBs and HBsAg in a total of 6950 students from four age groups were collected. The overall anti-HBs and HBsAg seropositivity rates were 44.3% and 1.2%, respectively. The anti-HBs seropositivity rate in the plasma-derived subgroup was significantly higher in both 15- and 18-year age groups. Overall response rate in the double-seronegative recipients at 15 years of age was 92.5% at 6 weeks following one recombinant HBV booster dose. Among the 24 recipients showing anti-HBs seroconversion at 6 weeks after booster, seven subjects (29.2%) had lost their anti-HBs seropositivity again within 3 years. Increased seropositivity rates and titers of anti-HBs did not provide additional protective effects among subjects comprehensively vaccinated against HBV in infancy.
Conclusions: HBV booster strategy at 15 years of age was the main contributor to the unique age-related phenomenon of anti-HBs seropositivity rate and titer. No increase in HBsAg seropositivity rates within different age groups was observed. Vaccination with plasma-derived HBV vaccines in infancy provided higher anti-HBs seropositivity at 15-18 years of age. Overall booster response rate was 92.5% and indicated that intact immunogenicity persisted at least 15 years after primary HBV vaccination in infancy. Booster vaccination of HBV did not confer additional protection against HBsAg carriage in our study.
Figures
References
Pre-publication history
-
- The pre-publication history for this paper can be accessed here: http://www.biomedcentral.com/1471-2458/14/991/prepub
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

