Smoking accelerates aging of the small airway epithelium
- PMID: 25248511
- PMCID: PMC4189169
- DOI: 10.1186/s12931-014-0094-1
Smoking accelerates aging of the small airway epithelium
Abstract
Background: Aging involves multiple biologically complex processes characterized by a decline in cellular homeostasis over time leading to a loss and impairment of physiological integrity and function. Specific cellular hallmarks of aging include abnormal gene expression patterns, shortened telomeres and associated biological dysfunction. Like all organs, the lung demonstrates both physiological and structural changes with age that result in a progressive decrease in lung function in healthy individuals. Cigarette smoking accelerates lung function decline over time, suggesting smoking accelerates aging of the lung. Based on this data, we hypothesized that cigarette smoking accelerates the aging of the small airway epithelium, the cells that take the initial brunt of inhaled toxins from the cigarette smoke and one of the primary sites of pathology associated with cigarette smoking.
Methods: Using the sensitive molecular parameters of aging-related gene expression and telomere length, the aging process of the small airway epithelium was assessed in age matched healthy nonsmokers and healthy smokers with no physical manifestation of lung disease or abnormalities in lung function.
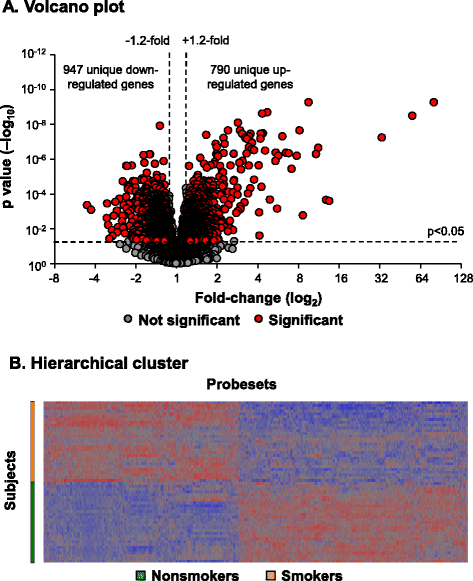
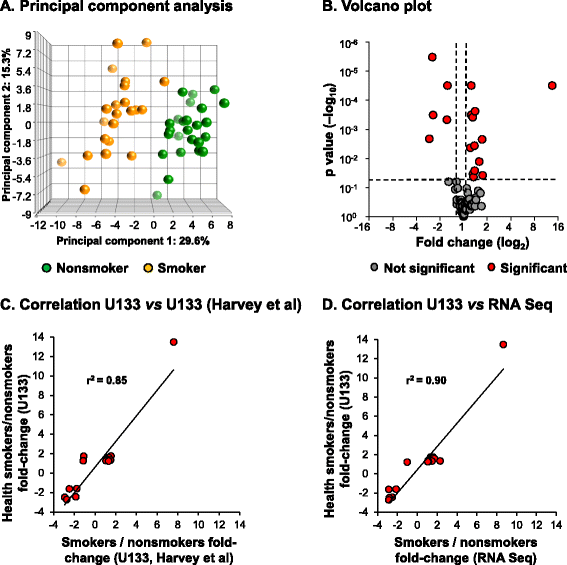
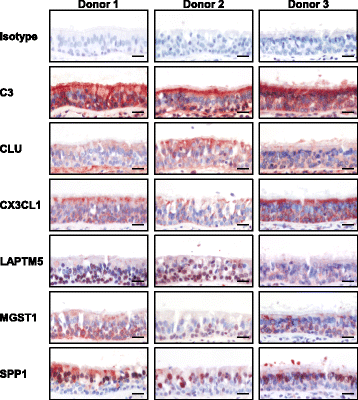
Results: Analysis of a 73 gene aging signature demonstrated that smoking significantly dysregulates 18 aging-related genes in the small airway epithelium. In an independent cohort of male subjects, smoking significantly reduced telomere length in the small airway epithelium of smokers by 14% compared to nonsmokers.
Conclusion: These data provide biologic evidence that smoking accelerates aging of the small airway epithelium.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

