Fluticasone propionate/salmeterol 250/50 μg versus salmeterol 50 μg after chronic obstructive pulmonary disease exacerbation
- PMID: 25248764
- PMCID: PMC4176847
- DOI: 10.1186/s12931-014-0105-2
Fluticasone propionate/salmeterol 250/50 μg versus salmeterol 50 μg after chronic obstructive pulmonary disease exacerbation
Abstract
Background: Inhaled long-acting beta2 agonists used alone and in combination with an inhaled corticosteroid reduce the risk of exacerbations in patients with stable COPD. However, the relative efficacy of these agents in preventing recurrent exacerbations in those recovering from an initial episode is not known. This study compared the rate of COPD exacerbations over the 26 weeks after an initial exacerbation in patients receiving the combination of fluticasone propionate and salmeterol (FP/SAL) or SAL alone.
Methods: Patients (n = 639) aged ≥40 years were randomized to either twice-daily inhaled FP/SAL 250/50 μg or SAL 50 μg. Primary, and secondary, endpoints were rates of recurrent severe, and moderate/severe, exacerbations of COPD. Lung function, health outcomes and levels of biomarkers of systemic inflammation were also assessed.
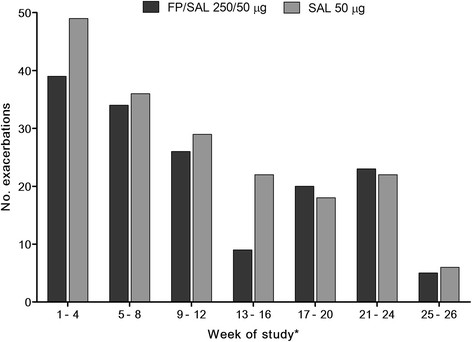
Results: There was no statistically significant treatment difference in rates of recurrent severe exacerbations (treatment ratio 0.92 [95% CI: 0.58, 1.45]) and moderate/severe exacerbations (0.82 [0.64, 1.06]) between FP/SAL and SAL in the intent-to-treat population. Pre-dose morning FEV1 change from baseline was greater (0.10 L [0.04, 0.16]) with FP/SAL than SAL. No treatment difference was seen for other endpoints including patient-reported health outcomes and biomarker levels for the full cohort.
Conclusions: No significant treatment difference between FP/SAL and SAL was seen in COPD exacerbation recurrence for the complete cohort. Treatment benefit with FP/SAL over SAL (treatment ratio 0.68 [0.47, 0.97]) was seen in patients having FEV1 ≥ 30% and prior exposure to ICS. No unexpected safety issues were identified with either treatment. Patients with the most severe COPD may be more refractory to treatment.
Trial registration: ClinicalTrials.gov (identifier NCT01110200). This study was funded by GlaxoSmithKline (study number ADC113874).
Figures




References
-
- Hurst JR, Vestbo J, Anzueto A, Locantore N, Müllerova H, Tal-Singer R, Miller B, Lomas DA, Agusti A, Macnee W, Calverley P, Rennard S, Wouters EF, Wedzicha JA, Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) Investigators Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363:1128–1138. doi: 10.1056/NEJMoa0909883. - DOI - PubMed
-
- Vestbo J, Edwards LD, Scanlon PD, Yates JC, Agusti A, Bakke P, Calverley PM, Celli B, Coxson HO, Crim C, Lomas DA, MacNee W, Miller BE, Silverman EK, Tal-Singer R, Wouters E, Rennard SI, ECLIPSE Investigators Changes in forced expiratory volume in 1 second over time in COPD. New Engl J Med. 2011;365:1184–1192. doi: 10.1056/NEJMoa1105482. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

