Membership and behavior of ultra-low-diversity pathogen communities present in the gut of humans during prolonged critical illness
- PMID: 25249279
- PMCID: PMC4173762
- DOI: 10.1128/mBio.01361-14
Membership and behavior of ultra-low-diversity pathogen communities present in the gut of humans during prolonged critical illness
Abstract
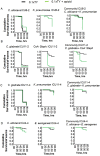
We analyzed the 16S rRNA amplicon composition in fecal samples of selected patients during their prolonged stay in an intensive care unit (ICU) and observed the emergence of ultra-low-diversity communities (1 to 4 bacterial taxa) in 30% of the patients. Bacteria associated with the genera Enterococcus and Staphylococcus and the family Enterobacteriaceae comprised the majority of these communities. The composition of cultured species from stool samples correlated to the 16S rRNA analysis and additionally revealed the emergence of Candida albicans and Candida glabrata in ~75% of cases. Four of 14 ICU patients harbored 2-member pathogen communities consisting of one Candida taxon and one bacterial taxon. Bacterial members displayed a high degree of resistance to multiple antibiotics. The virulence potential of the 2-member communities was examined in C. elegans during nutrient deprivation and exposure to opioids in order to mimic local conditions in the gut during critical illness. Under conditions of nutrient deprivation, the bacterial members attenuated the virulence of fungal members, leading to a "commensal lifestyle." However, exposure to opioids led to a breakdown in this commensalism in 2 of the ultra-low-diversity communities. Application of a novel antivirulence agent (phosphate-polyethylene glycol [Pi-PEG]) that creates local phosphate abundance prevented opioid-induced virulence among these pathogen communities, thus rescuing the commensal lifestyle. To conclude, the gut microflora in critically ill patients can consist of ultra-low-diversity communities of multidrug-resistant pathogenic microbes. Local environmental conditions in gut may direct pathogen communities to adapt to either a commensal style or a pathogenic style.
Importance: During critical illness, the normal gut microbiota becomes disrupted in response to host physiologic stress and antibiotic treatment. Here we demonstrate that the community structure of the gut microbiota during prolonged critical illness is dramatically changed such that in many cases only two-member pathogen communities remain. Most of these ultra-low-membership communities display low virulence when grouped together (i.e., a commensal lifestyle); individually, however, they can express highly harmful behaviors (i.e., a pathogenic lifestyle). The commensal lifestyle of the whole community can be shifted to a pathogenic one in response to host factors such as opioids that are released during physiologic stress and critical illness. This shift can be prevented by using compounds such as Pi-PEG15-20 that interrupt bacterial virulence expression. Taking the data together, this report characterizes the plasticity seen with respect to the choice between a commensal lifestyle and a pathogenic lifestyle among ultra-low-diversity pathogen communities that predominate in the gut during critical illness and offers novel strategies for prevention of sepsis.
Copyright © 2014 Zaborin et al.
Figures








References
-
- Alverdy J, Holbrook C, Rocha F, Seiden L, Wu RL, Musch M, Chang E, Ohman D, Suh S. 2000. Gut-derived sepsis occurs when the right pathogen with the right virulence genes meets the right host: evidence for in vivo virulence expression in Pseudomonas aeruginosa. Ann. Surg. 232:480–489. 10.1097/00000658-200010000-00003 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
