Modifying charge and hydrophilicity of simple Ru(II) polypyridyl complexes radically alters biological activities: old complexes, surprising new tricks
- PMID: 25249443
- PMCID: PMC4186668
- DOI: 10.1021/ic5013796
Modifying charge and hydrophilicity of simple Ru(II) polypyridyl complexes radically alters biological activities: old complexes, surprising new tricks
Abstract
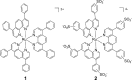
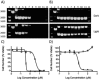
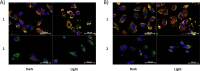
Compounds capable of light-triggered cytotoxicity are appealing potential therapeutics, because they can provide spatial and temporal control over cell killing to reduce side effects in cancer therapy. Two simple homoleptic Ru(II) polypyridyl complexes with almost-identical photophysical properties but radically different physiochemical properties were investigated as agents for photodynamic therapy (PDT). The two complexes were identical, except for the incorporation of six sulfonic acids into the ligands of one complex, resulting in a compound carrying an overall -4 charge. The negatively charged compound exhibited significant light-mediated cytotoxicity, and, importantly, the negative charges resulted in radical alterations of the biological activity, compared to the positively charged analogue, including complete abrogation of toxicity in the dark. The charges also altered the subcellular localization properties, mechanism of action, and even the mechanism of cell death. The incorporation of negative charged ligands provides a simple chemical approach to modify the biological properties of light-activated Ru(II) cytotoxic agents.
Figures


Similar articles
-
Critical Overview of the Use of Ru(II) Polypyridyl Complexes as Photosensitizers in One-Photon and Two-Photon Photodynamic Therapy.Acc Chem Res. 2017 Nov 21;50(11):2727-2736. doi: 10.1021/acs.accounts.7b00180. Epub 2017 Oct 23. Acc Chem Res. 2017. PMID: 29058879 Review.
-
Light-activated ruthenium complexes photobind DNA and are cytotoxic in the photodynamic therapy window.Chem Commun (Camb). 2012 Oct 7;48(77):9649-51. doi: 10.1039/c2cc33359g. Chem Commun (Camb). 2012. PMID: 22908094
-
Photoactive Ru(II) complexes with dioxinophenanthroline ligands are potent cytotoxic agents.Inorg Chem. 2014 Oct 6;53(19):10030-2. doi: 10.1021/ic5017164. Epub 2014 Sep 8. Inorg Chem. 2014. PMID: 25198057
-
Tuning the cytotoxic properties of new ruthenium(III) and ruthenium(II) complexes with a modified bis(arylimino)pyridine Schiff base ligand using bidentate pyridine-based ligands.J Biol Inorg Chem. 2014 Jun;19(4-5):675-89. doi: 10.1007/s00775-013-1083-4. Epub 2014 Jan 16. J Biol Inorg Chem. 2014. PMID: 24430199
-
Versatile Impact of Serum Proteins on Ruthenium(II) Polypyridyl Complexes Properties - Opportunities and Obstacles.Curr Protein Pept Sci. 2019;20(11):1052-1059. doi: 10.2174/1389203720666190513090851. Curr Protein Pept Sci. 2019. PMID: 31092177 Review.
Cited by
-
Combination of Ru(ii) complexes and light: new frontiers in cancer therapy.Chem Sci. 2015 May 1;6(5):2660-2686. doi: 10.1039/c4sc03759f. Epub 2015 Jan 13. Chem Sci. 2015. PMID: 29308166 Free PMC article.
-
Disruption of microtubule function in cultured human cells by a cytotoxic ruthenium(ii) polypyridyl complex.Chem Sci. 2019 Nov 18;11(1):264-275. doi: 10.1039/c9sc05671h. Chem Sci. 2019. PMID: 34040721 Free PMC article.
-
Avobenzone incorporation in a diverse range of Ru(II) scaffolds produces potent potential antineoplastic agents.Dalton Trans. 2020 Sep 15;49(35):12161-12167. doi: 10.1039/d0dt02016h. Dalton Trans. 2020. PMID: 32845256 Free PMC article.
-
The development of anticancer ruthenium(ii) complexes: from single molecule compounds to nanomaterials.Chem Soc Rev. 2017 Oct 2;46(19):5771-5804. doi: 10.1039/c7cs00195a. Chem Soc Rev. 2017. PMID: 28654103 Free PMC article. Review.
-
Tracking the cellular uptake and phototoxicity of Ru(ii)-polypyridyl-1,8-naphthalimide Tröger's base conjugates.RSC Chem Biol. 2024 Feb 21;5(4):344-359. doi: 10.1039/d3cb00206c. eCollection 2024 Apr 3. RSC Chem Biol. 2024. PMID: 38576718 Free PMC article.
References
-
- Lippert B.Cisplatin: Chemistry and Biochemistry of a Leading Anticancer Drug; Wiley–VCH: Weinheim, Germany, 1999.
-
- Cohen S. M.; Lippard S. J.. Prog. Nucleic Acid Res. Mol. Biol. 2001, 67, 93–130. - PubMed
-
- Hadjiliadis N.; Sletten E.. Metal Complex–DNA Interactions, 1st Edition; Wiley: Chichester, U.K., 2009.
-
- Moucheron C. New J. Chem. 2009, 332235–245.
-
- Boerner L. J. K.; Zaleski J. M. Curr. Opin. Chem. Biol. 2005, 92135–144. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

