Physiological effects and therapeutic potential of proinsulin C-peptide
- PMID: 25249503
- PMCID: PMC4254984
- DOI: 10.1152/ajpendo.00130.2014
Physiological effects and therapeutic potential of proinsulin C-peptide
Abstract
Connecting Peptide, or C-peptide, is a product of the insulin prohormone, and is released with and in amounts equimolar to those of insulin. While it was once thought that C-peptide was biologically inert and had little biological significance beyond its role in the proper folding of insulin, it is now known that C-peptide binds specifically to the cell membranes of a variety of tissues and initiates specific intracellular signaling cascades that are pertussis toxin sensitive. Although it is now clear that C-peptide is a biologically active molecule, controversy still remains as to the physiological significance of the peptide. Interestingly, C-peptide appears to reverse the deleterious effects of high glucose in some tissues, including the kidney, the peripheral nerves, and the vasculature. C-peptide is thus a potential therapeutic agent for the treatment of diabetes-associated long-term complications. This review addresses the possible physiologically relevant roles of C-peptide in both normal and disease states and discusses the effects of the peptide on sensory nerve, renal, and vascular function. Furthermore, we highlight the intracellular effects of the peptide and present novel strategies for the determination of the C-peptide receptor(s). Finally, a hypothesis is offered concerning the relationship between C-peptide and the development of microvascular complications of diabetes.
Keywords: C-peptide; diabetes; diabetes-associated complications.
Figures
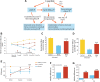
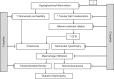
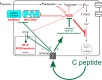

References
-
- Al-Rasheed NM, Meakin F, Royal EL, Lewington AJ, Brown J, Willars GB, Brunskill NJ. Potent activation of multiple signaling pathways by C-peptide in opossum kidney proximal tubular cells. Diabetologia 47: 987–997, 2004. - PubMed
-
- Al-Rasheed NM, Willars GB, Brunskill NJ. C-peptide signals via Galpha i to protect against TNF-alpha-mediated apoptosis of opossum kidney proximal tubular cells. J Am Soc Nephrol 17: 986–995, 2006. - PubMed
-
- Bathgate RA, Halls ML, van der Westhuizen ET, Callander GE, Kocan M, Summers RJ. Relaxin family peptides and their receptors. Physiol Rev 93: 405–480, 2013. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

