Maternal insulin resistance and transient hyperglycemia impact the metabolic and endocrine phenotypes of offspring
- PMID: 25249504
- PMCID: PMC4233258
- DOI: 10.1152/ajpendo.00210.2014
Maternal insulin resistance and transient hyperglycemia impact the metabolic and endocrine phenotypes of offspring
Abstract
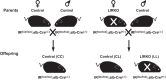
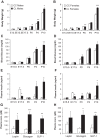
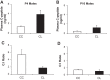
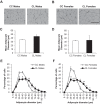
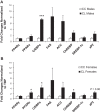
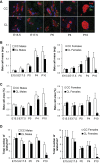
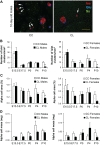
Studies in both humans and rodents suggest that maternal diabetes leads to a higher risk of the fetus developing impaired glucose tolerance and obesity during adulthood. However, the impact of hyperinsulinemia in the mother on glucose homeostasis in the offspring has not been fully explored. We aimed to determine the consequences of maternal insulin resistance on offspring metabolism and endocrine pancreas development using the LIRKO mouse model, which exhibits sustained hyperinsulinemia and transient increase in blood glucose concentrations during pregnancy. We examined control offspring born to either LIRKO or control mothers on embryonic days 13.5, 15.5, and 17.5 and postpartum days 0, 4, and 10. Control offspring born to LIRKO mothers displayed low birth weights and subsequently rapidly gained weight, and their blood glucose and plasma insulin concentrations were higher than offspring born to control mothers in early postnatal life. In addition, concentrations of plasma leptin, glucagon, and active GLP-1 were higher in control pups from LIRKO mothers. Analyses of the endocrine pancreas revealed significantly reduced β-cell area in control offspring of LIRKO mothers shortly after birth. β-Cell proliferation and total islet number were also lower in control offspring of LIRKO mothers during early postnatal days. Together, these data indicate that maternal hyperinsulinemia and the transient hyperglycemia impair endocrine pancreas development in the control offspring and induce multiple metabolic alterations in early postnatal life. The relatively smaller β-cell mass/area and β-cell proliferation in these control offspring suggest cell-autonomous epigenetic mechanisms in the regulation of islet growth and development.
Keywords: endocrine pancreas development; hyperinsulinemia; intrauterine environment; maternal insulin resistance; offspring metabolism.
Copyright © 2014 the American Physiological Society.
Figures

Similar articles
-
[Effects of severe hyperglycaemia in pregnancy and early overfeeding on islet development and insulin resistance].Zhonghua Fu Chan Ke Za Zhi. 2010 Sep;45(9):658-63. Zhonghua Fu Chan Ke Za Zhi. 2010. PMID: 21092544 Chinese.
-
Prenatal Testosterone Exposure Leads to Gonadal Hormone-Dependent Hyperinsulinemia and Gonadal Hormone-Independent Glucose Intolerance in Adult Male Rat Offspring.Biol Reprod. 2016 Jan;94(1):5. doi: 10.1095/biolreprod.115.133157. Epub 2015 Nov 19. Biol Reprod. 2016. PMID: 26586841 Free PMC article.
-
Maternal hypothyroidism in mice influences glucose metabolism in adult offspring.Diabetologia. 2020 Sep;63(9):1822-1835. doi: 10.1007/s00125-020-05172-x. Epub 2020 May 30. Diabetologia. 2020. PMID: 32472193 Free PMC article.
-
Offspring body size and metabolic profile - effects of lifestyle intervention in obese pregnant women.Dan Med J. 2014 Jul;61(7):B4893. Dan Med J. 2014. PMID: 25123127 Review.
-
Impact of maternal diabetes on epigenetic modifications leading to diseases in the offspring.Exp Diabetes Res. 2012;2012:538474. doi: 10.1155/2012/538474. Epub 2012 Nov 22. Exp Diabetes Res. 2012. PMID: 23227034 Free PMC article. Review.
Cited by
-
The physiological basis with uterine myometrium contractions from electro-mechanical/hormonal myofibril function to the term and preterm labor.Heliyon. 2023 Nov 19;9(11):e22259. doi: 10.1016/j.heliyon.2023.e22259. eCollection 2023 Nov. Heliyon. 2023. PMID: 38034762 Free PMC article. Review.
-
A short-term transition from a high-fat diet to a normal-fat diet before pregnancy exacerbates female mouse offspring obesity.Int J Obes (Lond). 2016 Apr;40(4):564-72. doi: 10.1038/ijo.2015.236. Epub 2015 Nov 26. Int J Obes (Lond). 2016. PMID: 26607040 Free PMC article.
-
Fetal hyperglycemia acutely induces persistent insulin resistance in skeletal muscle.J Endocrinol. 2019 Jul 1;242(1):M1-M15. doi: 10.1530/JOE-18-0455. J Endocrinol. 2019. PMID: 30444716 Free PMC article.
-
The Effect of a Maternal Mediterranean Diet in Pregnancy on Insulin Resistance is Moderated by Maternal Negative Affect.Nutrients. 2020 Feb 6;12(2):420. doi: 10.3390/nu12020420. Nutrients. 2020. PMID: 32041106 Free PMC article.
-
Metabolites and metabolic pathways associated with glucocorticoid resistance in pregnant African-American women.Compr Psychoneuroendocrinol. 2020 Feb-May;1-2:100001. doi: 10.1016/j.cpnec.2020.100001. Epub 2020 Mar 30. Compr Psychoneuroendocrinol. 2020. PMID: 33693436 Free PMC article.
References
-
- Barbour LA, McCurdy CE, Hernandez TL, Kirwan JP, Catalano PM, Friedman JE. Cellular mechanisms for insulin resistance in normal pregnancy and gestational diabetes. Diabetes Care 30, Suppl 2: S112–S119, 2007. - PubMed
-
- Barnes SK, Ozanne SE. Pathways linking the early environment to long-term health and lifespan. Prog Biophys Mol Biol 106: 323–336, 2011. - PubMed
-
- Biddinger SB, Kahn CR. From mice to men: insights into the insulin resistance syndromes. Annu Rev Physiol 68: 123–158, 2006. - PubMed
-
- Bursztyn M, Gross ML, Goltser-Dubner T, Koleganova N, Birman T, Smith Y, Ariel I. Adult hypertension in intrauterine growth-restricted offspring of hyperinsulinemic rats: evidence of subtle renal damage. Hypertension 48: 717–723, 2006. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

