2-[(4,6-Di-amino-pyrimidin-2-yl)sulfan-yl]-N-(2-methyl-phen-yl)acetamide
- PMID: 25249901
- PMCID: PMC4158502
- DOI: 10.1107/S1600536814015256
2-[(4,6-Di-amino-pyrimidin-2-yl)sulfan-yl]-N-(2-methyl-phen-yl)acetamide
Abstract
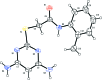
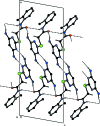
In the title compound, C13H15NOS, the plane of the pyrimidine ring makes a dihedral angle of 54.73 (9)° with that of the o-tolyl ring. The mol-ecule adopts an extended conformation, which is evident from the C-C(=O)-N-Car (ar = aromatic) torsion angle of 178.42 (15)°. In the crystal, mol-ecules are linked via pairs of N-H⋯N hydrogen bonds, forming inversion dimers with an R (2) 2(8) ring motif. The dimers are linked by N-H⋯O and C-H⋯O hydrogen bonds, with the O atom accepting three such interactions, forming sheets parallel to (100).
Keywords: crystal structure.
Figures
References
-
- Bruker (2008). APEX2, SAINT and SADABS Bruker AXS Inc., Madison, Wisconsin, USA.
-
- Farrugia, L. J. (2012). J. Appl. Cryst. 45, 849–854.
-
- Hocková, D., Holý, A., Masojídková, M., Andrei, G., Snoeck, R., De Clercq, E. & Balzarini, J. (2003). J. Med. Chem. 46, 5064–5073. - PubMed
-
- Hocková, D., Holý, A. N., Masojídková, M., Andrei, G., Snoeck, R., De Clercq, E. & Balzarini, J. (2004). Bioorg. Med. Chem. 12, 3197–3202. - PubMed
-
- Perales, J. B., Freeman, J., Bacchi, C. J., Bowling, T., Don, R., Gaukel, E., Mercer, L., et al. (2011). Bioorg. Med. Chem. Lett. 21, 2816–2819. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
