C1-Inhibitor protects from focal brain trauma in a cortical cryolesion mice model by reducing thrombo-inflammation
- PMID: 25249935
- PMCID: PMC4158993
- DOI: 10.3389/fncel.2014.00269
C1-Inhibitor protects from focal brain trauma in a cortical cryolesion mice model by reducing thrombo-inflammation
Abstract
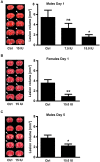
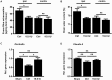
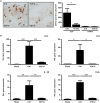
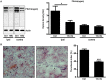
Traumatic brain injury (TBI) induces a strong inflammatory response which includes blood-brain barrier damage, edema formation and infiltration of different immune cell subsets. More recently, microvascular thrombosis has been identified as another pathophysiological feature of TBI. The contact-kinin system represents an interface between inflammatory and thrombotic circuits and is activated in different neurological diseases. C1-Inhibitor counteracts activation of the contact-kinin system at multiple levels. We investigated the therapeutic potential of C1-Inhibitor in a model of TBI. Male and female C57BL/6 mice were subjected to cortical cryolesion and treated with C1-Inhibitor after 1 h. Lesion volumes were assessed between day 1 and day 5 and blood-brain barrier damage, thrombus formation as well as the local inflammatory response were determined post TBI. Treatment of male mice with 15.0 IU C1-Inhibitor, but not 7.5 IU, 1 h after cryolesion reduced lesion volumes by ~75% on day 1. This protective effect was preserved in female mice and at later stages of trauma. Mechanistically, C1-Inhibitor stabilized the blood-brain barrier and decreased the invasion of immune cells into the brain parenchyma. Moreover, C1-Inhibitor had strong antithrombotic effects. C1-Inhibitor represents a multifaceted anti-inflammatory and antithrombotic compound that prevents traumatic neurodegeneration in clinically meaningful settings.
Keywords: C1-inhibitor; blood-brain barrier; contact-kinin system; edema; inflammation; thrombosis; traumatic brain injury.
Figures
References
-
- Albert-Weissenberger C., Stetter C., Meuth S. G., Gobel K., Bader M., Sirén A. L., et al. (2012). Blocking of bradykinin receptor B1 protects from focal closed head injury in mice by reducing axonal damage and astroglia activation. J. Cereb. Blood Flow Metab. 32, 1747–1756 10.1038/jcbfm.2012.62 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

